Researchers at Weill Cornell Medicine with an international team have used liver biopsies to identify cellular and molecular markers that can potentially be used to predict whether and when pancreatic cancer will spread to an individual’s liver or elsewhere, such as the lung.

Credit: Vanessa Dudley
Researchers at Weill Cornell Medicine with an international team have used liver biopsies to identify cellular and molecular markers that can potentially be used to predict whether and when pancreatic cancer will spread to an individual’s liver or elsewhere, such as the lung.
The study, published on June 28 in Nature Medicine, proposes that information from a liver biopsy—a small tissue sample collected for lab analysis—when pancreatic cancer is diagnosed may help guide doctors in personalizing treatment, such as liver-directed immunotherapies, before cancer cells have the chance to metastasize.
Only 10 percent of people with pancreatic cancer will survive more than two years after initial diagnosis. “If we can predict the timing and location of metastases, that could be a real game changer in treating pancreatic cancer, particularly patients at high metastatic risk,” said study co-senior author Dr. David Lyden, the Stavros S. Niarchos Professor in Pediatric Cardiology and professor of pediatrics and of cell and developmental biology at Weill Cornell Medicine.
In 2015, Dr. Lyden and his colleagues discovered that pancreatic cancer cells secrete factors that reach distant organs, most often the liver, to establish a pre-metastatic niche for new tumors to form.
To find out how these alterations prime their new location for cancerous colonization, Dr. Lyden collaborated with lead author Dr. Linda Bojmar, an adjunct assistant professor of molecular biology research in pediatrics at Weill Cornell Medicine and assistant professor of clinical and experimental medicine at Linköping University in Sweden.
Together with researchers at Memorial Sloan Kettering Cancer Center, including co-senior author Dr. William Jarnagin, co-first authors Drs. Constantinos Zambirinis and Jonathan Hernandez, and the hepatopancreatobiliary team, they obtained liver biopsies from 49 individuals who underwent surgical treatment for early-stage pancreatic cancer. They also collected liver biopsies from 19 people who underwent a similar operation for conditions unrelated to cancer, for example, removal of benign pancreatic cysts.
Liver Biopsies Reveal Early Signs of Rapid Metastasis
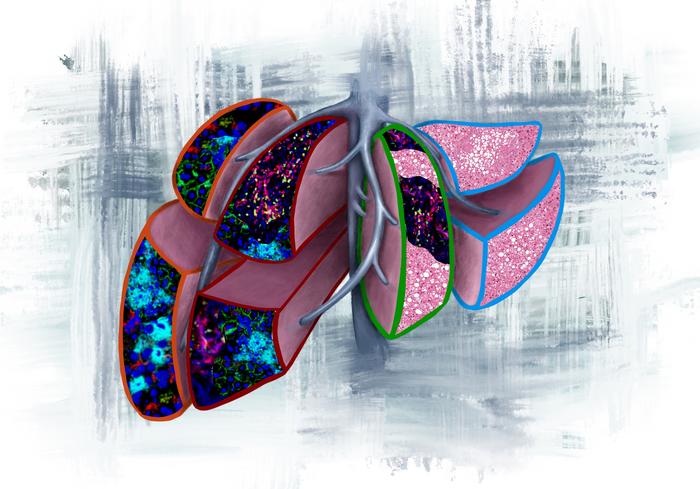
The researchers then conducted a battery of molecular, cellular and metabolic analyses on these samples to determine whether they could identify hallmarks that preceded—or potentially prevented—subsequent metastases in the patients. They found that the livers of recurrence-free survivors, who showed no signs of cancer spread after a follow-up period of at least three years, looked much like the livers of people who never had cancer.
At the other end of the spectrum were those who developed liver metastases within six months of diagnosis—a patient group that has poor prognosis with limited therapeutic options. Their livers were riddled with so-called neutrophil extracellular traps (NETs), dense tangles of DNA and enzymes released by dying neutrophils, immune cells that are a first line of defense against infection. Because these NETs are strongly associated with future metastases and develop so early in the course of disease, radiological imaging in the near future may be able to detect them and identify patients in danger of this aggressive spread.
“These individuals could then receive a full course of chemotherapy or, if the metastases are detected when only a few appear, perhaps the secondary tumors could be surgically removed,” said Dr. Lyden, who is also a member of the Sandra and Edward Meyer Cancer Center and the Gale and Ira Drukier Insitute for Children’s Health at Weill Cornell Medicine. In addition, he and his colleagues are investigating whether drugs that digest the DNA which forms the NETs could prevent liver metastases.
Immune Responses in Later Metastases
The researchers identified two other categories of patients in the study: those who would go on to develop later metastases to the liver and those who would have the cancer spread to other sites, such as the lung. Patients whose cancers spread to organs other than the liver, showed a strong immune response fighting the cancer—protective T cells and natural killer cells had infiltrated their livers, and many immune-regulatory genes were activated. These individuals who are prone to developing metastases outside the liver may benefit from immunotherapy to boost their ongoing anti-tumor immune response.
On the flip side, those whose livers succumbed to later metastases also accumulated immune cells—but the cells showed signs of metabolic exhaustion. “It’s as if the liver was trying to protect itself, but lost the battle in the end,” said Dr. Bojmar.
The researchers plan to validate their findings in a larger cohort of patients with pancreatic cancer and examine if this approach could be useful with other newly diagnosed cancers. “We hope to develop a tool for predicting which patients with colorectal cancer will go on to develop liver metastases based on the cellular, molecular and metabolic profiles of their liver biopsies,” said co-senior author Dr. Robert Schwartz, associate professor of medicine at Weill Cornell Medicine.
Authors from multiple institutes throughout the United States and in Sweden and Israel contributed to this study.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see profile for Dr. David Lyden.
This work was supported in part by: National Cancer Institute grants CA224175, CA210240, CA232093, CA163117, CA207983, CA163120, CA169416, CA169538, CA218513 and the National Institute of Allergy & Infectious Diseases grant AI144301; the United States Department of Defense grants W81XWH-13-1-0425, W81XWH-13-1-0427, W81XWH-13-1-0249, W81XWH-14-1-0199 and W81XWH-21-1-0978; the National Institutes of Health/National Center for Advancing Translational Sciences grant UL1TR002384; and the National Institutes of Health grants R01CA234614 and R01DK121072.
Journal
Nature Medicine