Ishikawa, Japan — Schizophrenia is a complicated mental health disorder accompanied by wide range of symptoms such as hallucinations, impaired cognitive ability, and disorganized speech or behavior. It has been associated with anomalies in neurotransmission due to the imbalance of chemical neurotransmitters. Current treatment strategies against schizophrenia involve the administration of antipsychotic drugs, which can cause adverse effects and are associated with a high risk of cardiovascular disease. Moreover, in patients, response to therapeutic drugs is often inadequate as the blood-brain barrier (BBB), a protective barrier of cells, strictly regulates the movement of ions and molecules into the brain.

Credit: Eijiro Miyako from JAIST.
Ishikawa, Japan — Schizophrenia is a complicated mental health disorder accompanied by wide range of symptoms such as hallucinations, impaired cognitive ability, and disorganized speech or behavior. It has been associated with anomalies in neurotransmission due to the imbalance of chemical neurotransmitters. Current treatment strategies against schizophrenia involve the administration of antipsychotic drugs, which can cause adverse effects and are associated with a high risk of cardiovascular disease. Moreover, in patients, response to therapeutic drugs is often inadequate as the blood-brain barrier (BBB), a protective barrier of cells, strictly regulates the movement of ions and molecules into the brain.
To overcome the hurdle of BBB and facilitate the transport of therapeutic drugs into brain tissue to treat schizophrenia, researchers have explored the applicability of receptor-mediated transcytosis (RMT) using low-density lipoprotein receptor-related protein 1 (LRP1). This research was conducted by team led by Associate Professor Eijiro Miyako from Japan Advanced Institute of Science and Technology (JAIST), included Prof. Yukio Ago from Hiroshima University, Prof. Shinsaku Nakagawa from Osaka University, Prof. Takatsugu Hirokawa from Tsukuba University, and Dr. Kotaro Sakamoto, Senior Principal Scientist at Ichimaru Pharcos Co., Ltd. Their study was published in JACS Au journal on June 20, 2024.
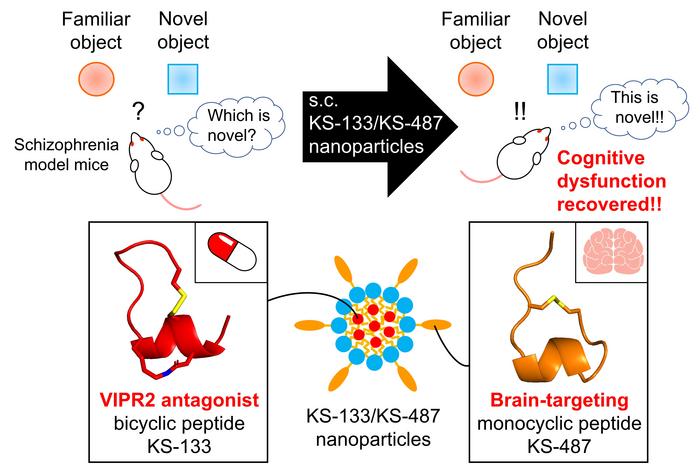
The researchers were inspired by previous findings which showed the interactions of vasoactive intestinal peptide receptor 2 (VIPR2) gene duplication in schizophrenia and their own discovery of a novel peptide, KS-133. The novel peptide, KS-133, has selective antagonist activity to VIPR2, leading to its downregulation. However, the major limitation associated with KS-133 was its poor permeability across BBB.
To facilitate the effective transport of KS-133 into the brain, they developed a brain-targeting peptide, KS-487, that could specifically bind to LRP1 and influence RMT. Finally, the researchers developed a novel nanoparticle-based drug delivery system (DDS) where KS-133 peptide was encapsulated with KS-487 targeting peptide and studied its efficacy in treating schizophrenia.
The administration of peptide formulations via the DDS resulted in the effective distribution of the drug in the brains of mice. Drug release profiles assessed by pharmacokinetic analysis confirmed the role of the brain-targeting peptide in transporting KS-133 into the brain. Furthermore, the efficacy of DDS was evaluated in mice with induced schizophrenia by elevated activation of VIPR2. Mice treated with KS-133/KS-487 nanoparticles showed significant improvement in cognitive functions during novel object recognition tests, which could be attributed to the inhibition of VIPR2.
Explaining the real-life applications and potential of their study, Dr. Miyako shares, “Existing drugs only have mechanisms involving neurotransmitter modulation, and their therapeutic effects are limited, especially for cognitive dysfunction. Thus, our peptide formulation could be used as a novel drug to restore cognitive dysfunction in schizophrenia.”
In summary, this study by Dr. Miyako and co-researchers provides preclinical evidence of a novel therapeutic strategy for targeting VIPR2 that could improve cognitive impairment in schizophrenia. “Going ahead, we will extend our study to involve cells and animal models, as well as human clinical trials, to confirm the efficacy and safety of this peptide formulation and promote its development as a new treatment for schizophrenia within 5 years,” concludes Dr. Miyako, optimistic about the long-term implications of their study.
We hope that the discovery and development of novel DDS utilizing bio-compatible peptides revolutionizes the treatment landscape of schizophrenia!
###
|
Title of original paper: |
Cyclic Peptides KS-133 and KS-487 Multifunctionalized Nanoparticles Enable Efficient Brain Targeting for Treating Schizophrenia |
|
Authors: |
Kotaro Sakamoto*, Seigo Iwata, Zihao Jin, Lu Chen, Tatsunori Miyaoka, Mei Yamada, Kaiga Katahira, Rei Yokoyama, Ami Ono, Satoshi Asano, Kotaro Tanimoto, Rika Ishimura, Shinsaku Nakagawa, Takatsugu Hirokawa, Yukio Ago*, and Eijiro Miyako* |
|
Journal: |
JACS Au |
|
DOI: |
10.1021/jacsau.4c00311 |
About Japan Advanced Institute of Science and Technology, Japan
Founded in 1990 in Ishikawa prefecture, the Japan Advanced Institute of Science and Technology (JAIST) was the first independent national graduate school in Japan. Now, after 30 years of steady progress, JAIST has become one of Japan’s top-ranking universities. JAIST counts with multiple satellite campuses and strives to foster capable leaders with a state-of-the-art education system where diversity is key; about 40% of its alumni are international students. The university has a unique style of graduate education based on a carefully designed coursework-oriented curriculum to ensure that its students have a solid foundation on which to carry out cutting-edge research. JAIST also works closely both with local and overseas communities by promoting industry–academia collaborative research.
About Associate Professor Eijiro Miyako from Japan Advanced Institute of Science and Technology, Japan
Dr. Miyako Eijiro is an Associate Professor at the Materials Chemistry Frontiers Research Area, Japan Advanced Institute of Science and Technology (JAIST). He has been a visiting scientist at Centre National de la Recherche Scientifique (CNRS) (France) and Nanyang Technological University (Singapore). He also served as the Senior Researcher at National Institute of Advanced Industrial Science and Technology (AIST), Japan. His research interests are in the areas of Bioengineering, Materials Chemistry, Nanotechnology, and Nanomedicine. Dr. Miyako received his Ph.D. in Chemical Systems and Engineering from Kyushu University (Japan) in 2006. He has received research prizes and awards such as PCCP Prize in Royal Society of Chemistry and Research Encouragement Award in The Fullerenes, Nanotubes and Graphene Research Society.
Funding information
This work was technically supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the Japan Agency for Medical Research and Development (AMED, JP18am0101114, JP23ama121026j0002) and Research Support Project for Life Science and Drug Discovery (BINDS) from AMED under grant numbers JP23ama121052 and JP23ama121054. Eijiro Miyako thanks the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (A) (Grant number 23H00551), JSPS KAKENHI Grant-in-Aid for Challenging Research (Pioneering) (Grant number 22K18440), the Japan Science and Technology Agency for Adaptable and Seamless Technology Transfer Program through Target-driven R&D (Grant Number JPMJTR22U1), Institute for Fermentation, Osaka, and the Uehara Memorial Foundation. This work was partially supported by grants from JSPS KAKENHI to Satoshi Asano [23K091380] and Yukio Ago [20H03392] and Tokyo Biochemical Research Foundation in the form of a grant to Yukio Ago. This research was also supported in part by AMED in the form of a grant to Yukio Ago [JP22ym0126809]. This work was partly supported by JST SPRING by a grant to Ami Ono (JPMJSP2132) and Seigo Iwata (JPMJSP2102).
Journal
JACS Au
Article Title
Cyclic Peptides KS-133 and KS-487 Multifunctionalized Nanoparticles Enable Efficient Brain Targeting for Treating Schizophrenia
Article Publication Date
20-Jun-2024