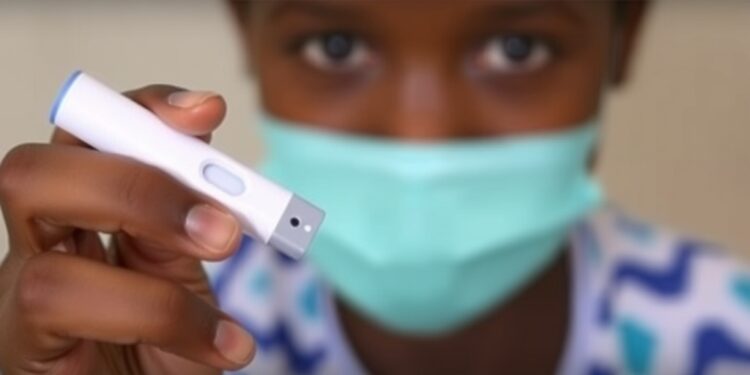
Researchers at Tulane University have made a groundbreaking advancement in the fight against tuberculosis (TB) with the development of a new handheld diagnostic device. This innovative tool is designed to deliver rapid and accurate diagnoses of TB in under an hour. The findings, documented in a study published in the prestigious journal Science Translational Medicine, underscore the urgency and necessity for improved diagnostic methods, particularly in low- and middle-income nations where TB prevalence is alarmingly high.
The device, compact and smartphone-sized, utilizes a lab-in-tube assay (LIT) technology that is both battery-powered and cost-effective. It stands as the first of its kind to identify Mycobacterium tuberculosis (Mtb) DNA from saliva samples—an addition to traditional blood and sputum sample testing. The ability to non-invasively collect saliva, particularly for testing children, addresses a significant gap in TB diagnostics. Children suffer especially from this infectious disease, with over a million diagnosed annually yet more than half remaining undiagnosed or unreported, according to the World Health Organization. The LIT device was meticulously crafted to make testing simpler and more efficient in rural and resource-limited settings, thereby potentially saving countless lives.
The ramifications of TB as a public health crisis cannot be overstated. Globally, TB is considered the deadliest infectious disease, infecting approximately 10 million people each year. This rising tide of infection, correlated with disruptions in routine healthcare services, emphasizes the urgent need for effective diagnostic tools that can be deployed in frontline health facilities. The director of the Tulane Center for Cellular & Molecular Diagnostics, Dr. Tony Hu, highlighted that the development of this affordable, simple testing platform is critical in managing TB, aiding not just in treating affected individuals but also in controlling the transmission of the disease within communities. The stark reality is that in 2021 alone, an estimated 4.2 million TB cases were either undiagnosed or unreported, a situation largely attributed to financial and operational constraints in high-burden areas.
Current diagnostic methods for TB are often marred by several challenges—including the high costs associated with existing technologies, which can exceed $19,000 for machinery, and roughly $100 per test. In contrast, the LIT device offers an innovative approach, attainable at a fraction of the cost: each device is priced at under $800, and testing costs about $3 per patient. This economic advantage positions the LIT device as a vital option for governments and organizations striving to implement health interventions in impoverished regions.
In clinical settings, the LIT device has shown remarkable results. In particular, during trials conducted with blood samples from children in the Dominican Republic, the system achieved 81% sensitivity, significantly surpassing the conventional device used, which produced only a 68% sensitivity rate. Notably, this is especially relevant for pediatric patients and individuals living with HIV, who frequently have difficulty producing sputum samples necessary for more traditional testing. The study’s findings not only validate the efficacy of blood serum-based testing but also indicate that these samples can adequately reflect a patient’s improvement during TB treatment.
The implications of utilizing saliva for testing are substantial. Brady Youngquist, the lead author of the study, pointed out that this system decreases the need for specialized technical knowledge and expensive equipment, making it well-suited for point-of-care applications. Saliva-based testing dramatically simplifies the collection process, allowing healthcare providers to perform tests without requiring a blood draw. This is particularly beneficial for vulnerable populations like children and HIV patients, who may struggle to produce the necessary sputum samples for conventional tests.
As the LIT technology continues to evolve, researchers are optimistic that it not only will improve the efficiency of TB diagnosis but also hold promise for other infectious diseases. The mobility and accessibility of the lab-in-tube model mean that testing could be conducted in more remote areas where traditional laboratory facilities are often unavailable. Such ability could make a monumental difference in controlling TB outbreaks and allowing for quicker interventions for those diagnosed.
Furthermore, the innovation reflects a growing recognition within the scientific community of the need for low-cost, high-impact diagnostic tools. The development team anticipates that as the LIT device undergoes further testing and refinement, it may set a new standard for diagnostic approaches in global health. The fight against TB can no longer afford to rely solely on established, costly methods; rather, it necessitates the integration of novel solutions that cater to the specific challenges faced in various healthcare environments.
While the LIT device is a significant step forward, additional efforts will be essential to expand access to testing and treatment for TB. A comprehensive strategy that encompasses diagnostics, medical care, and preventive measures is crucial to reducing the burden of this disease. Collaboration across governmental, non-governmental, and private sectors will be necessary to ensure that innovations like the LIT device are implemented effectively, reaching at-risk populations and yielding tangible health outcomes.
The road ahead is undeniably challenging, but advancements like the LIT device provide hope in advancing the global agenda against TB. Research teams around the world are now tasked with measuring the impact of this technology, honing the device for broader use, and addressing the disparities that contribute to the global TB epidemic. Ultimately, as we harness scientific innovation, our collective ability to make strides against TB will hinge upon ensuring that every individual has access to the diagnostic tools and treatments they need.
With ongoing support from various health organizations, including the National Institutes of Health and the U.S. Department of Defense, research into affordable approaches to TB diagnosis continues to be a strategic priority. This pioneering research underscores the evolving landscape of tuberculosis treatment and prevention—an urgent reminder of the important link between cutting-edge science and practical solutions for global health issues.
As the TB landscape shifts in the age of innovation, the drive for accessible, rapid testing systems like the LIT device could be pivotal in turning the tide against an infectious disease that has claimed millions of lives. The commitment from the research community and echoed support from public health entities highlights that innovative efforts, grounded in scientific inquiry, may lead to groundbreaking changes in the way we approach global health challenges.
In conclusion, while formidable barriers to TB diagnosis and treatment remain, the introduction of this novel handheld device signifies a hopeful advancement in medical technology. The potential lives saved, particularly among vulnerable populations, is a compelling testament to the power of research-based innovation. With sustained efforts, the fight against tuberculosis can evolve to meet the challenges of today and tomorrow.
Subject of Research: Handheld Diagnostic Device for Tuberculosis
Article Title: Rapid tuberculosis diagnosis from respiratory or blood samples by a low cost, portable lab-in-tube assay
News Publication Date: 9-Apr-2025
Web References: http://dx.doi.org/10.1126/scitranslmed.adp6411
References: Science Translational Medicine
Image Credits: Tulane University
Keywords: Tuberculosis, Diagnostic Device, Handheld Technology, Global Health, Public Health, Saliva Testing, Point-of-Care Testing, Mycobacterium tuberculosis, Children and TB, Biomedical Innovation.