In the field of orthopedic medicine, one of the most challenging issues continues to be the management of infected bone defects (IBDs), a condition that can significantly impede recovery and lead to severe complications. Recent innovations in biomaterials offer new hope for clinicians and patients alike, particularly concerning the development of bone regeneration scaffolds designed to stimulate bone growth while combating infections. A pioneering study published in the journal BME Frontiers introduces a novel dual-functional scaffold named Qx-D, which demonstrates remarkable potential in the treatment of infected bone defects.
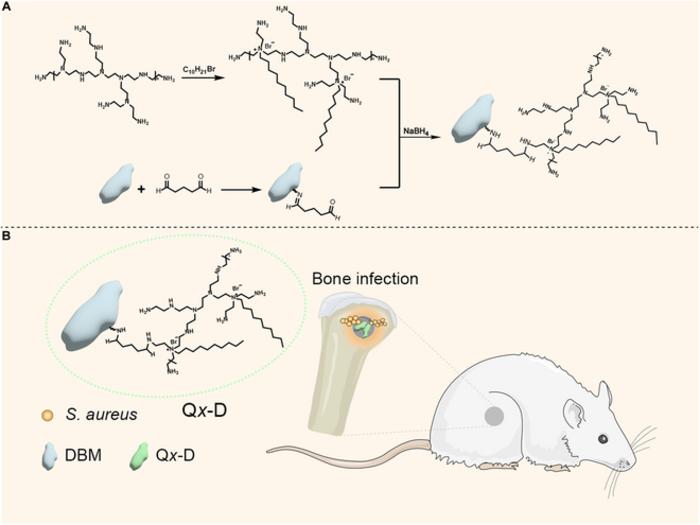
This research aims to enhance early intervention and surgical outcomes for patients suffering from bone infections. The focus of the study is on engineered demineralized bone matrix (DBM), a highly regarded, naturally derived biomaterial recognized for its osteogenic properties. Despite its advantages, DBM alone lacks inherent antimicrobial activity, rendering it vulnerable to infections post-surgery. In a bid to overcome this limitation, the research team modified the DBM by incorporating a macromolecular quaternary ammonium salt, known as QPEI.
The Qx-D scaffolds were synthesized via a simple yet effective Schiff base reaction, allowing for the fine-tuning of their composition through varied feeding ratios. The results were promising, revealing that the Qx-D scaffolds exhibit potent antibacterial properties against a wide spectrum of bacteria, including both Gram-positive strains like Staphylococcus aureus, as well as Gram-negative strains such as Escherichia coli. Notably, the antibacterial efficiency of the Qx-D scaffold reached an astonishing 99.9%, indicating significant effectiveness in combating infections that can compromise bone repair.
In terms of biocompatibility, the Qx-D scaffolds performed exceptionally well in vitro. The scaffolds were shown to facilitate the adhesion and differentiation of bone marrow stromal cells (BMSCs), which play a pivotal role in the process of bone regeneration. Furthermore, alkaline phosphatase (ALP) staining demonstrated that the Qx-D scaffolds positively influenced the osteogenic differentiation of BMSCs without adversely affecting cell viability or activity. Such findings underscore the scaffold’s potential as a viable option in regenerative medicine.
The in vivo efficacy of the Qx-D scaffolds was investigated through experiments conducted on a rat model specifically designed to mimic infected bone defects. The findings were encouraging, revealing that implantation of the Qx-D scaffold significantly mitigated inflammation and stimulated bone regeneration. Micro-computed tomography (CT) imaging illustrated a near-complete closure of the defects in the treatment group that received the Qx-D scaffold, with a notably higher bone volume/total volume (BV/TV) ratio when compared to the control group. These results strongly support the scaffold’s capability to restore structural integrity in compromised bone.
The implications of the study are profound, as the introduction of dual-functional biomaterials like Qx-D could redefine treatment paradigms for infected bone defects. Traditional methods frequently rely on long courses of antibiotics, which may lead to adverse effects such as antibiotic resistance—an issue of growing concern worldwide. The innovative design of the Qx-D scaffold, comprising both osteogenic and antibacterial characteristics, presents a compelling alternative that could significantly reduce dependency on antibiotics while enhancing patient outcomes.
As bone infections pose a significant risk to individuals facing orthopedic surgeries, the potential of Qx-D to transform treatment approaches is noteworthy. The research team highlights that, with continued development and thorough clinical trials, this novel scaffold could establish itself as a standard care option for patients suffering from infected bone defects. Such advancements would not only improve recovery trajectories for patients but also alleviate the substantial burden of bone infection management that healthcare systems currently face.
In conclusion, the development of the Qx-D scaffold represents a paradigm shift in the treatment of infected bone defects. The combination of enhanced osteogenic properties and robust antibacterial activity positions this scaffold at the forefront of orthopedic regenerative strategies. As researchers continue to refine these innovations, the hope for improved clinical outcomes grows stronger, with the possibility of revolutionizing the care provided to patients with challenging bone infections.
The exciting findings from this study lay the groundwork for future innovations in biomaterial science, encouraging ongoing exploration in the quest for next-generation orthopedic solutions. With the promising results obtained from early trials, the Qx-D scaffold could soon find its way into clinical practice, providing new avenues for recovery to those impacted by infected bone defects. The journey of research is ongoing, but one thing is clear—the future of orthopedic treatment is bright with the advent of novel biomaterials like Qx-D.
Subject of Research: Infected Bone Defects Treatment
Article Title: Cationized Decalcified Bone Matrix for Infected Bone Defect Treatment
News Publication Date: 2-Oct-2024
Web References: BME Frontiers (DOI: 10.34133/bmef.0066)
References: Not provided
Image Credits: Fu-Jian Xu Lab@BUCT
Keywords
Bone diseases, Bone formation, Bacterial infections, Biomaterials, Regenerative medicine