Photocatalytic hydrogen evolution from water is a key technology for achieving sustainable hydrogen production. However, the direct impact of the microscopic structure of interfacial water molecules on photocatalytic reactivity remains unexplored. In this study, the crucial roles of interfacial hydrogen bond structure and dynamics, as well as the optimal interfacial water environment for promoting H2 evolution were uncovered. These findings provide molecular-level insights that can guide the design of interfacial water conditions to enhance photocatalytic performance.

Credit: Zhongqiu LIN, Toshiki Sugimoto
Photocatalytic hydrogen evolution from water is a key technology for achieving sustainable hydrogen production. However, the direct impact of the microscopic structure of interfacial water molecules on photocatalytic reactivity remains unexplored. In this study, the crucial roles of interfacial hydrogen bond structure and dynamics, as well as the optimal interfacial water environment for promoting H2 evolution were uncovered. These findings provide molecular-level insights that can guide the design of interfacial water conditions to enhance photocatalytic performance.
Hydrogen production via photocatalytic water splitting is a sustainable solution for next-generation energy by utilizing light energy at room temperature. However, the design of innovative photocatalysts remains a challenge due to a limited molecular-level understanding of interfacial water molecules and their hydrogen bond networks. Unveiling the physicochemical properties of these interfacial water molecules is critical to optimizing photocatalytic efficiency and achieving breakthroughs in sustainable hydrogen production.
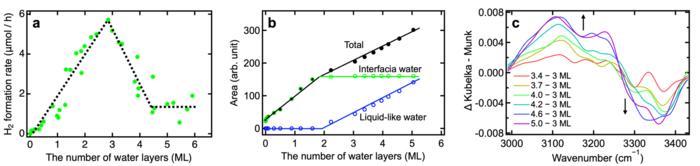
Researchers (Zhongqiu Lin et al.) led by Toshiki Sugimoto, Associate Professor at Institute for Molecular Science / The Graduate University for Advanced Studies, SOKENDAI, have comprehensively investigated the impact of interfacial H-bond networks using various TiO2 photocatalysts and uncovered a crucial role of interfacial H-bond structure/dynamics and optimal interfacial water environment for H2 evolution. They controlled the thickness of adsorbed water from sub-monolayer to multilayers by precisely adjusting water vapor pressure. With this approach, they succeeded in directly demonstrating the correlation between H2 formation rate and the microscopic structure of H-bond networks using real-time mass spectrometry and infrared absorption spectroscopy (Figure 1). Regardless of the crystalline structure of the TiO2 photocatalyst (brookite, anatase, or a mixture of anatase and rutile), they observed a linear increase in H2 formation rate with water adsorption up to three layers (Figure 1a), indicating that reactive water molecules are present not only in the first adsorbed layer but also in several overlying layers. However, the H2 formation rate turned to decrease dramatically when more than three layers of water covered the TiO2 surface (Figure 1a). In this situation, infrared spectra clearly indicated two distinct types of adsorbed water on the TiO2 surface: interfacial water and liquid-like water (Figure 1b). Due to many-body interactions among adsorbed water molecules, the liquid-like water adsorbed in more than three layers led to strengthening of interfacial H-bond (Figure 1c), which hinder interfacial proton-coupled hole transfer and drastically decreased the H2 formation rate. Based on these microscopic insights, their study suggests that depositing three water layers in a water vapor environment is optimal for photocatalytic hydrogen evolution (Figure 2).
Photocatalysis has been extensively studied for over half a century, predominantly in aqueous solution environments. In this context, this study represents a potential paradigm shift, demonstrating the effectiveness of water vapor environments compared to traditional liquid-phase reaction systems. These findings open new avenues for the molecular-level design and engineering of interfacial water toward the development of more innovative photocatalytic systems for next-generation renewable energy production.
Paper Information:
Authors: Zhongqiu Lin, Hikaru Saito, Hiromasa Sato, and Toshiki Sugimoto
Journal Name: Journal of the American Chemical Society
Journal Title: “Positive and Negative Impacts of Interfacial Hydrogen Bonds on Photocatalytic Hydrogen Evolution”
DOI: 10.1021/jacs.4c04271
Journal
Journal of the American Chemical Society
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Positive and Negative Impacts of Interfacial Hydrogen Bonds on Photocatalytic Hydrogen Evolution
Article Publication Date
5-Jul-2024