Immobilizing small synthetic molecules inside protein crystals proves to be a promising avenue for studying intermediate compounds formed during chemical reactions, report scientists from Tokyo Tech. By integrating this method with time-resolved serial femtosecond crystallography, they successfully visualized reaction dynamics and rapid structural changes occurring within reaction centers immobilized inside protein crystals. This innovative strategy holds significant potential for the intelligent design of drugs, catalysts, and functional materials.

Credit: Tokyo Institute of Technology
Immobilizing small synthetic molecules inside protein crystals proves to be a promising avenue for studying intermediate compounds formed during chemical reactions, report scientists from Tokyo Tech. By integrating this method with time-resolved serial femtosecond crystallography, they successfully visualized reaction dynamics and rapid structural changes occurring within reaction centers immobilized inside protein crystals. This innovative strategy holds significant potential for the intelligent design of drugs, catalysts, and functional materials.
Most complex chemical reactions, whether synthetic or biological, do not involve a direct transformation of reactants into products. Instead, they often proceed through the formation of short-lived intermediate compounds that undergo further reactions until the final products are obtained. Understanding these stepwise processes in detail is crucial for advancements in fields such as energy generation, catalysis, and medicine.
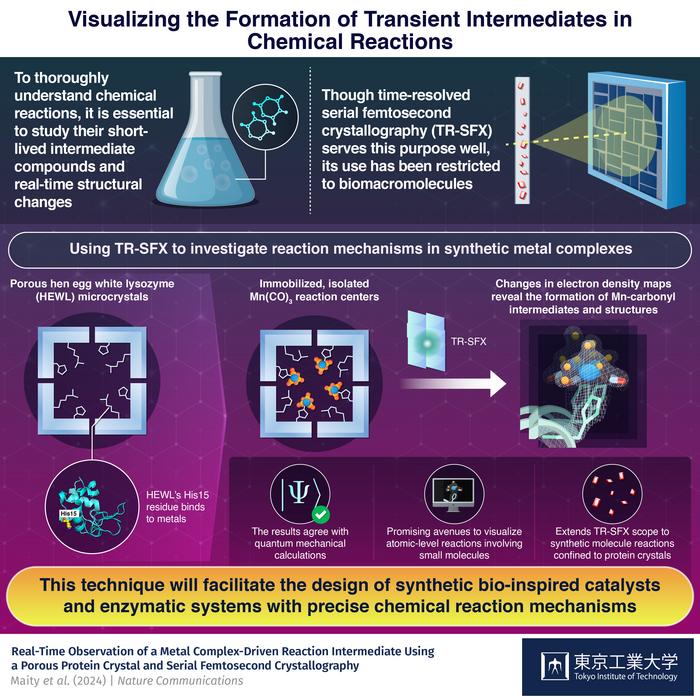
However, visualizing short-lived intermediates in chemical reactions is quite challenging, especially if one needs to capture structural changes in a molecule at the atomic level. One cutting-edge method to achieve this is time-resolved serial femtosecond crystallography (TR-SFX). This technique involves shooting extremely fast electron laser pulses at crystalized molecular structures and capturing the ensuing diffraction patterns. These molecular structures are in various stages of a chemical reaction, allowing the reaction dynamics to be recorded. Despite its astonishing capabilities, the use of TR-SFX thus far has been mostly limited to biomacromolecules.
To extend the applications of this powerful technique and showcase its potential for other types of molecules, a research team from Japan, led by Professor Takafumi Ueno, decided to use it to analyze reactions in synthetic compounds. Using an innovative approach, they successfully captured the dynamics of carbon monoxide (CO) release from Mn(CO)3. Their study was published on
One significant limitation that the researchers had to overcome is the fact that TR-SFX works best with microcrystals, such as those formed by biomacromolecules. Additionally, although small molecules can form crystals suitable for TR-SFX, these crystals are generally tightly packed, leaving little room for reactions to occur.
To address these issues, the team developed an innovative strategy based around hen egg-white lysozyme (HEWL). This naturally occurring protein not only crystallizes into a nanoporous structure suitable for TR-SFX, but also contains His15 terminals that bind strongly to metals. The researchers took advantages of these properties to immobilize a light-sensitive Mn(CO)3-containing compound inside HEWL crystals. This setup provided a suitable environment for studying CO release reactions, which were triggered by shooting light pulses at the crystals at carefully controlled time intervals relative to the TR-SFX electron laser pulses.
Overall, this protocol proved highly promising for investigating the target reaction by analyzing changes in electron density maps obtained via TR-SFX. “After CO release, Mn centers typically undergo dimerization, aerial oxidation or precipitation in solution, which complicates mechanistic investigations. In our work, by isolating the Mn reaction centers in a restricted protein environment, we enabled a detailed experimental analysis of the intermediates generated during the progression of the reaction,” explains Ueno.
Worth noting, the experimental results were in excellent agreement with quantum mechanical calculations, validating the proposed strategy. “We have established that, by using protein crystals as a matrix, the reactions of synthetic metal complex can be studied, including the determination of any intermediate structures,” concludes Ueno, “This advancement holds the potential to facilitate the design of artificial metalloenzymes with precise mechanisms, empowering the design, control, and development of innovative reactions.”
In conclusion, the method developed in this work provides an avenue for the smart design of new drugs, catalysts, and enzymatic systems involving non-biological components. Let us hope this new tool pushes many applied fields further towards next-generation technologies!
###
About Tokyo Institute of Technology
Tokyo Tech stands at the forefront of research and higher education as the leading university for science and technology in Japan. Tokyo Tech researchers excel in fields ranging from materials science to biology, computer science, and physics. Founded in 1881, Tokyo Tech hosts over 10,000 undergraduate and graduate students per year, who develop into scientific leaders and some of the most sought-after engineers in industry. Embodying the Japanese philosophy of “monotsukuri,” meaning “technical ingenuity and innovation,” the Tokyo Tech community strives to contribute to society through high-impact research.
Journal
Nature Communications
Method of Research
Experimental study
Article Title
Real-time observation of a metal complex-driven reaction intermediate using a porous protein crystal and serial femtosecond crystallography
Article Publication Date
29-Jun-2024