In the relentless battle against cancer, understanding the microscopic environment in which tumors thrive is crucial. A groundbreaking study led by researchers at The University of Osaka has unveiled a paradoxical mechanism by which oxygen deficiency within colon tumors actually fuels their growth. Challenging long-held assumptions, this discovery shines new light on the complex relationship between hypoxia—localized oxygen deprivation—and tumor progression, potentially reshaping future therapeutic strategies against colon cancer.
Traditionally, hypoxia within tumors was viewed as a hostile condition, one that restricted cancer growth by limiting oxygen-dependent cellular processes. In fact, many anticancer approaches have targeted this very vulnerability by attempting to starve tumors of blood supply and oxygen. However, clinical outcomes have often been unpredictable, with some treatments inadvertently accelerating tumor growth instead of suppressing it. This conundrum has puzzled oncologists and molecular biologists alike, urging deeper exploration into hypoxia’s nuanced role in tumor biology.
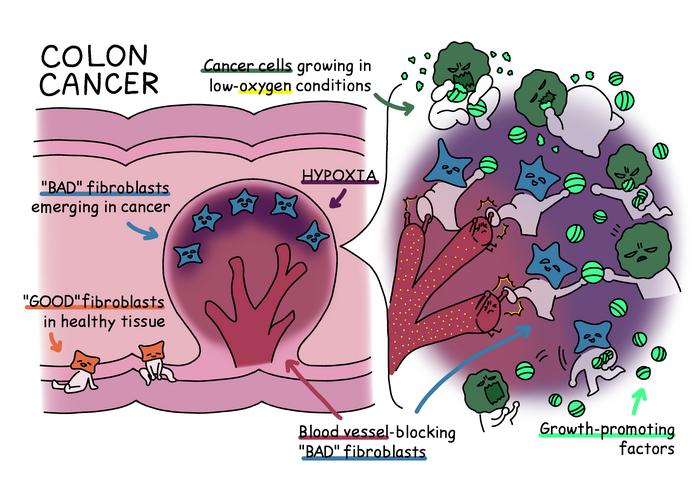
The recent study, published in Nature Communications, elucidates a critical pathway whereby hypoxic conditions within the tumor microenvironment induce a transformative shift in local fibroblast cells. Fibroblasts are connective tissue cells normally responsible for maintaining structural integrity and supporting healthy tissue function. Intriguingly, in oxygen-depleted niches near the tumor surface, these fibroblasts undergo a malignant reprogramming into a pro-inflammatory, tumor-promoting phenotype. These “bad” fibroblasts not only adapt to the harsh hypoxic environment but exploit it, becoming architects of a microenvironment that accelerates oncogenesis.
Mechanistically, these transformed fibroblasts begin secreting two pivotal molecules: epiregulin and Wnt5a. Epiregulin acts as a potent growth factor that stimulates cancer cell proliferation, while Wnt5a plays an instrumental role in reinforcing the hypoxic state by inhibiting angiogenesis—the formation of new blood vessels. This inhibition prevents oxygen from flooding the tumor microenvironment, thereby sustaining the very hypoxia that triggered the fibroblastic transformation. This self-perpetuating cycle creates a niche optimized for tumor survival and expansion, turning the hypoxic microenvironment from a presumed adversary into an accomplice of malignancy.
To validate these findings, the research team employed both murine models and human tissue samples, encompassing healthy colon, inflammatory bowel disease, and colon cancer specimens. Remarkably, the pattern of fibroblast transformation and Wnt5a secretion was consistent across species and disease states, underscoring the universal relevance of this mechanism. This cross-validation strengthens the argument that targeting Wnt5a-secreting fibroblasts could represent a novel therapeutic avenue that complements existing modalities targeting cancer and immune cells.
The discovery is of particular significance given colon cancer’s prevalence as the leading type of malignancy in Japan, and indeed worldwide. By revealing the dual role of hypoxia—as both a stressor and an enabler of tumor progression—this work compels the scientific community to reevaluate current anti-angiogenic therapies, which may inadvertently catalyze cancer growth through unintended hypoxia-induced fibroblast activation.
Furthermore, these insights extend beyond oncology. Fibroblast activation and hypoxia interplay are also implicated in chronic inflammatory disorders such as inflammatory bowel disease. Understanding the cellular and molecular underpinnings of fibroblast behavior in hypoxic states could therefore inform novel treatment strategies for these debilitating conditions, which currently lack fully effective therapies.
Akira Kikuchi, the senior author of the study, emphasized the translational potential of these findings. “By targeting the Wnt5a-producing inflammatory fibroblasts, we open the door to therapies that disrupt this malignant microenvironmental feedback loop, offering hope for more effective management of colon cancer,” he stated. This approach heralds a shift toward a tri-modal therapeutic paradigm, integrating cancer cells, immune responses, and stromal fibroblast dynamics.
Technically, the experimental design involved precise spatial mapping of oxygen levels within tumor masses, identification of fibroblast subpopulations through lineage tracing, and comprehensive gene expression analyses. Advanced imaging and quantitative assays confirmed that areas with pronounced hypoxia corresponded to regions rich in inflammatory fibroblasts expressing epiregulin and Wnt5a, reinforcing the causal link between oxygen deprivation and fibroblast-mediated tumor promotion.
Moreover, the study’s innovative use of both animal and human data sets exemplifies the power of translational research, bridging fundamental discoveries with clinical relevance. The similarity in fibroblast behavior across species validates murine models as effective platforms for preclinical investigation of fibroblast-targeted therapies, accelerating the trajectory from bench to bedside.
This paradigm-shifting research recalibrates the understanding of tumor microenvironment dynamics, showcasing how intrinsic cellular actors adapt and manipulate pathophysiological conditions to favor cancer progression. As such, the identification of hypoxia-induced Wnt5a-secreting fibroblasts not only enhances scientific comprehension but also stimulates the pursuit of targeted interventions that may improve survival outcomes for colon cancer patients globally.
In conclusion, this study underscores the importance of microenvironmental context in cancer biology and the versatile roles of non-cancerous stromal cells. The notion that hypoxia, once regarded solely as a limitation to cancer growth, can paradoxically accelerate malignancy through fibroblast-mediated mechanisms presents a compelling narrative that will undoubtedly influence future cancer research and treatment paradigms.
Subject of Research: Animal tissue samples
Article Title: Hypoxia-induced Wnt5a-secreting fibroblasts promote colon cancer progression
News Publication Date: 17-Apr-2025
Web References: https://doi.org/10.1038/s41467-025-58748-9
Image Credits: Osakana Funwari
Keywords: Health and medicine, Cancer, Colon cancer, Cancer immunology, Inflammatory bowel diseases, Cancer treatments, Drug therapy, Disease progression, Pathophysiology