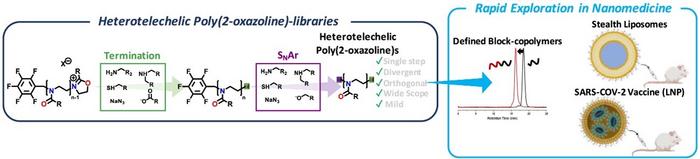
A collaboration between Leiden University (Assistant Prof. Joachim F. R. Van Guyse) and the Innovation Center for NanoMedicine (iCONM; Center Director: Prof. Kazunori Kataoka), a research institute of the Kawasaki Institute of Industrial Promotion (KIIP), has announced that they devised a new way to quickly and reliably diversify the reactive end-groups on poly(2-oxazoline)s, a biocompatible polymer class. Their approach enables quick exploration of poly(2-oxazoline)s in nanomedicine applications, whereby it can replace poly(ethylene glycol) (PEG), allowing to tune pharmacokinetics of nanomedicine and provide potential solutions for patients with contraindications against PEG.
Reactive end-groups on non-immunogenic biocompatible polymers, such as PEG, are frequently used in the synthesis of nanomedicine, whereby the biocompatible polymer improves the stability, blood circulation time, thus enabling passive accumulation at neovascular sites, whilst avoiding premature blood clearance and off-target toxicity. The installment of a biocompatible ‘stealth’ polymer is common practice in the field, often dubbed ‘PEGylation’, having led to FDA-approved PEG-protein conjugates (e.g. Peginterferon alfa-2a), PEGylated liposomes (e.g. Doxil), and more recently the lipid nanoparticle (LNP)-mediated SARS-COV-2 vaccines (e.g. Comirnaty, BioNTech/Pfizer). These technologies heavily rely on the production of PEG with reactive end-groups, which are obtained through multistep synthetic procedures. Poly(2-oxazoline)s (POx), a class of biocompatible polymers with large structural versatility, are being investigated as a potential alternative to PEG, whereby their structure can be fine-tuned to modulate the pharmacokinetics and -dynamics, whilst avoiding PEG-specific immune responses in patients. Despite these promising properties, the generation of libraries comprising POx with two different reactive chain-ends was often tedious from a synthetic standpoint, requiring iterative synthesis of components, or chemistry with a relatively limited scope, partially impeding wide-spread POxylation. To facilitate straightforward end-group diversification of POx, the authors employed commercially available pentafluorobenzyl bromide or tosylate initiators for the polymerization, whereby the termination step could be selectively performed with O-, N- and S-nucleophiles, followed by a subsequent para-fluoro nucleophilic aromatic substitution of the pentafluorobenzyl group with O-, N-, S-nucleophiles. Due to the broad substrate scope, various functional moieties could be easily introduced, which is attractive for the engineering of nano-sized drug/gene delivery platforms. The authors demonstrated that their approach enabled the rapid synthesis of POx-lipid conjugates, which were explored in liposomes and LNP-mediated mRNA delivery, whereby the introduced plurifluorophenyl-linker had a negligible effect on their performance. Encouraged by these results, these lipids were explored in the administration of SARS-COV-2 spike mRNA and compared to their PEGylated counterparts, whereby both displayed robust immune responses, highlighting the potential of POx as a promising PEG alternative.

Credit: J. Van Guyse
A collaboration between Leiden University (Assistant Prof. Joachim F. R. Van Guyse) and the Innovation Center for NanoMedicine (iCONM; Center Director: Prof. Kazunori Kataoka), a research institute of the Kawasaki Institute of Industrial Promotion (KIIP), has announced that they devised a new way to quickly and reliably diversify the reactive end-groups on poly(2-oxazoline)s, a biocompatible polymer class. Their approach enables quick exploration of poly(2-oxazoline)s in nanomedicine applications, whereby it can replace poly(ethylene glycol) (PEG), allowing to tune pharmacokinetics of nanomedicine and provide potential solutions for patients with contraindications against PEG.
Reactive end-groups on non-immunogenic biocompatible polymers, such as PEG, are frequently used in the synthesis of nanomedicine, whereby the biocompatible polymer improves the stability, blood circulation time, thus enabling passive accumulation at neovascular sites, whilst avoiding premature blood clearance and off-target toxicity. The installment of a biocompatible ‘stealth’ polymer is common practice in the field, often dubbed ‘PEGylation’, having led to FDA-approved PEG-protein conjugates (e.g. Peginterferon alfa-2a), PEGylated liposomes (e.g. Doxil), and more recently the lipid nanoparticle (LNP)-mediated SARS-COV-2 vaccines (e.g. Comirnaty, BioNTech/Pfizer). These technologies heavily rely on the production of PEG with reactive end-groups, which are obtained through multistep synthetic procedures. Poly(2-oxazoline)s (POx), a class of biocompatible polymers with large structural versatility, are being investigated as a potential alternative to PEG, whereby their structure can be fine-tuned to modulate the pharmacokinetics and -dynamics, whilst avoiding PEG-specific immune responses in patients. Despite these promising properties, the generation of libraries comprising POx with two different reactive chain-ends was often tedious from a synthetic standpoint, requiring iterative synthesis of components, or chemistry with a relatively limited scope, partially impeding wide-spread POxylation. To facilitate straightforward end-group diversification of POx, the authors employed commercially available pentafluorobenzyl bromide or tosylate initiators for the polymerization, whereby the termination step could be selectively performed with O-, N- and S-nucleophiles, followed by a subsequent para-fluoro nucleophilic aromatic substitution of the pentafluorobenzyl group with O-, N-, S-nucleophiles. Due to the broad substrate scope, various functional moieties could be easily introduced, which is attractive for the engineering of nano-sized drug/gene delivery platforms. The authors demonstrated that their approach enabled the rapid synthesis of POx-lipid conjugates, which were explored in liposomes and LNP-mediated mRNA delivery, whereby the introduced plurifluorophenyl-linker had a negligible effect on their performance. Encouraged by these results, these lipids were explored in the administration of SARS-COV-2 spike mRNA and compared to their PEGylated counterparts, whereby both displayed robust immune responses, highlighting the potential of POx as a promising PEG alternative.
What is the novelty of this study?
Functional, biocompatible, water-soluble polymers are basic components in therapeutic compounds or formulations, enabling improved drug/gene delivery, safety profile due to reduced side effects, or decreasing the necessary administration frequency. Poly(2-oxazoline)s, a biocompatible polymer class with large structural versatility, enable the fine-tuning of pharmacokinetic and -dynamic properties, though the exploration in nanomedicine thereof is partially hampered by the lack of accessible end-group diversification strategies.
• A simple, single-step end-group diversification approach is presented, relying on the orthogonal reactivity of an electrophilic pentafluorobenzyl group and an electrophilic 2-oxazolinium species, the reactive chain-end in the polymerization of POx.
• The approach enables synthetic diversification with a large number of commercially available substrates, namely O-, N-, and S-nucleophiles, whilst featuring excellent end-group fidelity and control over the molecular weight distribution. A 25-membered library of heterotelechelic POx is shown.
• The approach enabled rapid exploration in the synthesis of platforms for nanomedicine, which was exemplified by the synthesis of POx-based block-copolymers, liposomes, and lipid nanoparticles for mRNA delivery.
• The POx-based lipid nanoparticles were comparable in transfection capability to their PEGylated counterparts. Also, the prophylactic effect in SARS-COV-2 vaccination was not compromised versus a PEG-control, highlighting both the opportunities of this polymer platform and the presented chemistry.
Why are these findings important and how is your study going to improve the current therapy?
The study is of importance as it facilitates the rapid development of POx-based nanomedicine platforms through a straightforward end-group diversification strategy, whereby the synthesized products conform to the strict quality criteria of commercial PEG products. Consequently, POxylated nanomedicine can be rapidly explored, allowing the careful tuning of pharmaceutical properties through the selection of the polymer structure.
Journal
Angewandte Chemie International Edition
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Facile generation of heterotelechelic poly(2-oxazoline)s towards accelerated exploration of poly(2-oxazoline)-based nanomedicine
Article Publication Date
23-Apr-2024