In the ongoing battle against breast cancer, precision in diagnostic methods remains paramount. A groundbreaking study recently published in BMC Cancer introduces an innovative approach to enhance the sensitivity of HER2 immunohistochemistry (IHC), a critical test used worldwide to guide personalized breast cancer therapy. This novel technique addresses longstanding challenges in HER2 detection by overcoming the physical constraints imposed by HER2 protein structures, heralding a new era for more accurate cancer diagnostics.
HER2, or human epidermal growth factor receptor 2, is a well-known biomarker in breast cancer. Detection of HER2 overexpression via IHC informs treatment decisions, including the use of targeted therapies like trastuzumab and pertuzumab, which significantly improve patient prognosis. However, despite advances in antibody technology, variability remains a concern. The inconsistency often stems from the structural complexity of HER2 in its different forms—particularly its existence as both monomers and dimers within cancer cells. These conformational states can obscure antibody binding sites, potentially leading to under-detection or misclassification of HER2 status, which profoundly affects clinical outcomes.
The innovative strategy detailed in this study pivots on the structural analysis of HER2 and its interaction with existing diagnostic antibodies. The researchers meticulously aligned the molecular configuration of HER2 heterodimers with the binding regions of trastuzumab and pertuzumab. Remarkably, both antibodies targeted nearly identical regions of the HER2 molecule, suggesting a steric hindrance—spatial interference—that could limit antibody accessibility when HER2 is in dimeric form. This insight catalyzed the search for alternative molecular probes capable of circumventing this limitation.
Taking inspiration from nature’s toolkit, the investigators turned to HER2-binding affibodies and nanobodies, small engineered proteins known for their high affinity and unique binding capabilities at sites distinct from traditional antibodies. They designed a fusion protein combining these two entities, termed Nby-Aby, capable of simultaneously targeting separate regions on the HER2 receptor. This dual-targeting mechanism ensures that the fusion protein binds effectively even when HER2 molecules dimerize, reducing the impact of steric hindrance that hampers conventional antibody flexibility.
To further enhance detection, the researchers incorporated these binding proteins into human heavy chain ferritin (HFn) nanoparticles, creating novel constructs such as Nby-HFn and Aby-HFn. These nanoparticles serve as scaffolds presenting multiple binding moieties, thereby increasing avidity and detection robustness. The use of ferritin-based nanoparticles, with their biocompatibility and structural stability, offers a promising platform for improving diagnostic reagents’ performance within the complex environment of tissue samples.
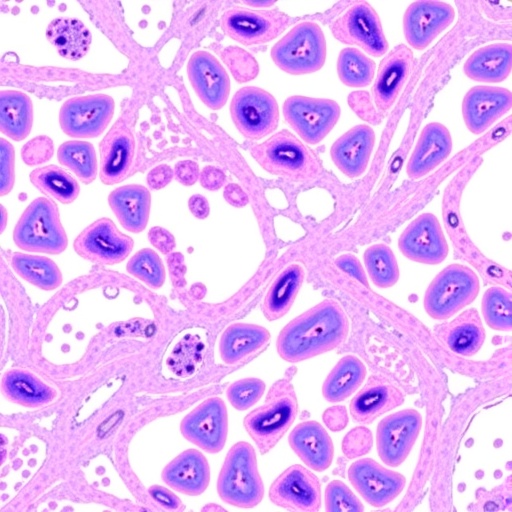
Validation of this avant-garde technology was performed using breast cancer tissue microarrays (TMAs), offering a high-throughput format to compare the new Nby-Aby assay against conventional HER2 antibodies. The results were illuminating: the dual-targeting Nby-Aby assay demonstrated substantially enhanced sensitivity in detecting HER2-positive cells across a broad spectrum of tissue samples. Enhanced detection was particularly notable in cases previously categorized as HER2-low or negative, suggesting that some tumors might be underdiagnosed using existing methodologies.
This refined detection capacity transcends mere incremental improvement—it signals a paradigm shift in how diagnostics can harness molecular engineering to tackle fundamental biological challenges. The dual-targeting principle effectively unveils HER2 epitopes occluded in dimeric formations, shedding light on cancer cells that may have evaded accurate classification. This not only impacts initial diagnosis but also has downstream implications for treatment stratification, patient monitoring, and outcome prediction.
The study’s findings challenge the current one-size-fits-all approach to IHC assays by underscoring the need to address protein conformation dynamics during antibody-based detection. By leveraging the natural specificity and modularity of nanobodies and affibodies, the researchers present a scalable and versatile platform adaptable to other receptor systems that may suffer from similar steric challenges.
Beyond diagnostic enhancement, this work opens the door to the potential development of therapeutic agents that exploit dual-binding mechanisms. Such agents could more effectively interfere with HER2 signaling pathways by simultaneously engaging multiple receptor sites, potentially overcoming resistance mechanisms linked to receptor dimerization. Moreover, nanoparticles like HFn could serve as delivery vehicles, combining diagnostic and therapeutic functionalities into so-called theranostic agents.
Importantly, the methodological rigor demonstrated in this study plays a pivotal role in its translational promise. Detailed structural alignments informed the design of fusion proteins, while robust immunohistochemical assessments across numerous patient-derived samples validated clinical relevance. This integrative approach, weaving molecular biophysics with clinical pathology, exemplifies the interdisciplinary innovation needed in cancer research.
The implications of improved HER2 detection extend beyond breast cancer. HER2 aberrations occur in other malignancies such as gastric, ovarian, and lung cancers, where precise biomarker evaluation critically guides therapy. The methodological advancements in overcoming steric hindrance and dual-targeting can potentially be adapted to these tumor types, amplifying the impact of this discovery.
From a technical perspective, the utilization of nanobodies derived from camelid antibodies offers excellent tissue penetration due to their small size, while affibodies provide high affinity and specificity. Their combination in a single fusion protein capitalizes on these complementary strengths, setting a precedent for next-generation diagnostic reagents. Furthermore, ferritin nanoparticles offer a robust and biocompatible scaffold, which could facilitate enhanced signal amplification in IHC staining protocols.
The researchers’ ability to significantly elevate HER2 scores in tissue microarrays compared to traditional antibody detection indicates that false negatives in current diagnostic workflows might be more common than previously acknowledged. Consequently, implementing such enhanced detection strategies could refine patient selection for HER2-targeted therapies, ultimately improving clinical outcomes through personalized medicine.
In conclusion, this pioneering study underscores the value of molecular engineering and structural biology insights in addressing long-standing diagnostic challenges. The novel dual-targeting Nby-Aby fusion protein and its nanoparticle conjugates represent a formidable advance in HER2 IHC testing. By overcoming the steric hindrance inherent in dimeric HER2 receptors, this approach enhances detection sensitivity, offering the promise of more accurate diagnostics and better-informed treatment decisions for breast cancer patients worldwide.
As this technology moves toward broader clinical adoption, further exploration into its applicability across diverse cancer types and integration with existing diagnostic platforms will be critical. The melding of molecular precision with clinical pathology heralds a new chapter in cancer diagnostics, one that promises to improve lives through smarter detection and tailored therapies.
Subject of Research: HER2 immunohistochemistry (IHC) detection improvement in breast cancer diagnosis through dual-targeting and steric hindrance resolution.
Article Title: Dual-targeting and steric hindrance resolution in HER2 IHC: a novel approach to improve diagnostic sensitivity
Article References:
Luo, L., Zhang, X., Chen, L. et al. Dual-targeting and steric hindrance resolution in HER2 IHC: a novel approach to improve diagnostic sensitivity. BMC Cancer 25, 1231 (2025). https://doi.org/10.1186/s12885-025-14553-7
Image Credits: Scienmag.com