In a groundbreaking advancement for the treatment of metastatic breast cancer, researchers at Sun Yat-sen Memorial Hospital in China have unveiled compelling results from a phase II clinical trial that investigates the effectiveness of combining pyrotinib, an irreversible tyrosine kinase inhibitor, with fulvestrant, a selective estrogen receptor degrader. This combination therapy has shown to significantly enhance clinical outcomes for patients diagnosed with hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer, a patient group that has limited treatment options following progression on trastuzumab-based therapies.
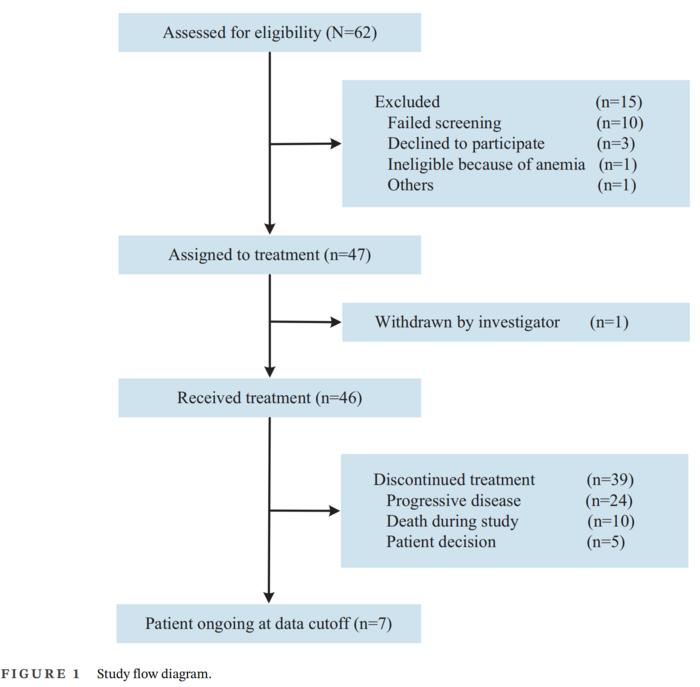
Initially, the study screened a total of 62 patients across five different hospital centers for eligibility, ultimately enrolling 46 patients who met the required criteria. Comprehensive evaluations revealed that a number of patients were excluded for various reasons, including failure to meet screening benchmarks, personal choice to decline participation, and specific medical ineligibilities, such as the presence of anemia. These 46 patients proceeded to receive a regimen of daily oral pyrotinib at a dosage of 400 mg alongside intramuscular injections of fulvestrant at 500 mg, conforming strictly to the study’s protocol.
The primary objective of the trial was to evaluate the efficacy and safety of this novel combination treatment. Results were promising and underscored the potential of this regimen to address a critical need in the treatment landscape of metastatic breast cancer. The median progression-free survival (PFS) for all participants reached an impressive 18.2 months, while patients in their first line of treatment realized a median PFS of 19.5 months. Notably, those with brain metastases, a subgroup historically difficult to treat, also achieved a median PFS of 18.4 months, suggesting that this combination may offer effective outcomes even in more complex cases.
Additionally, the disease control rate (DCR) reported from the trial was remarkable, indicating that 97.5% of enrolled patients exhibited disease stability, with 32.5% of responders achieving objective responses, including both complete and partial responses. This level of efficacy is particularly crucial in the context of HR+/HER2+ metastatic breast cancer, as these patients typically struggle with treatment options after progression on conventional therapies.
In terms of tolerability, the safety profile of the combined treatment was encouraging. Throughout the trial, no grade 4 or higher adverse events were reported, which highlights the regimen’s potential for a favorable therapeutic window. The most common grade 3 adverse event noted was diarrhea, occurring in 26.1% of patients, but it was effectively managed with supportive care strategies. The absence of severe toxicities suggests that this combination could be a viable chemotherapy-free option for patients who are resistant to more traditional treatments.
An integral aspect of this trial was the exploration of biomarkers that might predict patient responses to the combination therapy. Researchers noted that specific genetic markers, such as low tumor mutation burden (TMB) and ZNF217 mutations, were associated with enhanced responsiveness to the treatment. This discovery underscores the growing relevance of precision medicine in oncology, where patient-specific factors are taken into account to tailor treatment approaches, ultimately improving outcomes based on biological markers.
The findings present pyrotinib and fulvestrant not only as effective alternatives to existing therapeutic regimens but also establish their position as potential first-line treatments for patients with HR+/HER2+ metastatic breast cancer. The researchers are planning to build upon these promising findings by initiating a phase III randomized controlled trial. This future study aims to compare the efficacy of this combination regimen against established standard first-line therapies, potentially reshaping treatment paradigms for this patient population.
Internet-enabled technologies, particularly artificial intelligence, are anticipated to play a role in advancing this research. AI can optimize patient selection and facilitate real-time monitoring of trial outcomes, providing more nuanced insights into the efficacy and safety profiles of emerging therapeutic approaches. As research progresses, the integration of advanced technologies could dramatically speed up the pace of drug development and improve clinical trial methodologies.
The high stakes of metastatic breast cancer cannot be underestimated. With thousands of patients entering into progressive disease states every year, the urgency for innovative solutions is paramount. Studies like this offer a beacon of hope for patients who have exhausted their therapeutic options, affirming that new avenues for treatment are on the horizon. The synergy of pyrotinib and fulvestrant represents a landmark in the fight against breast cancer, and their successful application could redefine clinical practice guidelines for the management of HR+/HER2+ metastatic breast cancer.
In summary, the results of this phase II trial not only signify that combining pyrotinib and fulvestrant may offer a promising course of action for patients with metastatic breast cancer, particularly those resistant to trastuzumab, but also herald a new era for treating complex cancer types. Ultimately, these advancements pave the way for larger scale studies and emphasize the importance of continual research and innovation in oncology.
Subject of Research: Combination of pyrotinib and fulvestrant for metastatic breast cancer
Article Title: Combined pyrotinib and fulvestrant for hormone receptor-positive and HER2-positive metastatic breast cancer: A multicenter, single-arm, phase II trial
News Publication Date: 20-Dec-2024
Web References: doi.org/10.1002/mco2.70031
References:
Image Credits: Ying Wang
Keywords
Metastatic breast cancer, Pyrotinib, Fulvestrant, Clinical trial, Targeted therapy, Precision medicine, Oncology, Hormone receptor-positive, HER2-positive, Progression-free survival, Disease control rate, Biomarkers.