In the relentless battle against hepatocellular carcinoma (HCC), a devastating primary liver cancer responsible for nearly 90% of all liver cancer cases worldwide, the scientific community continues to seek clarity on the molecular orchestrators that fuel its onset and aggressive progression. A significant breakthrough has recently emerged from a comprehensive multi-institutional study spearheaded by researchers affiliated with Harbin Medical University, Bishan Hospital of Chongqing Medical University, and Chongqing Medical University. This pioneering work sheds new light on the enigmatic role of the protein-coding gene C1orf122 in exacerbating HCC, unveiling intricate mechanisms that position this molecule as both a potent biomarker and a promising therapeutic target.
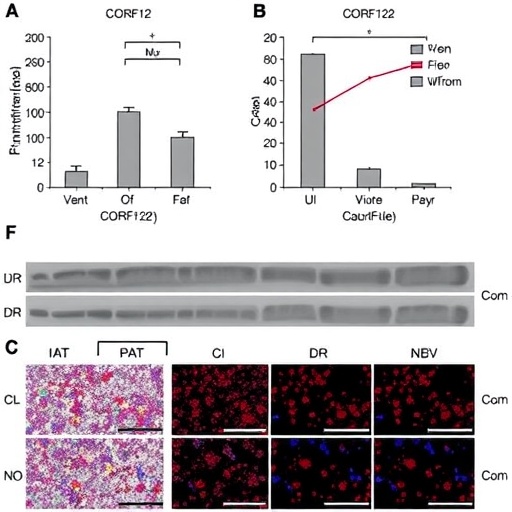
Hepatocellular carcinoma represents a formidable challenge due to its multifactorial etiology and complex pathogenesis, often eluding early detection and effective intervention. Building upon a growing body of evidence that implicates chromosome 1 open reading frame 122 (C1orf122) in various oncogenic pathways, the study adopts an integrative approach, employing extensive data mining of the TCGA pan-cancer database complemented by rigorous experimental validations. Results reveal a compelling overexpression of C1orf122 in HCC tumor tissues relative to their normal counterparts—an aberrant molecular signature that correlates strongly with poorer overall survival rates in patients, accentuating its prognostic value.
Delving deeper into functional assays, the research delineates the causative impact of C1orf122 overexpression on HCC cell biology. Cell viability and proliferative assays highlight a pronounced increase in the growth capacity of HepG2 and HuH-7 hepatoma cells upon C1orf122 upregulation, while targeted gene knockdown triggers a substantial reduction in these malignant properties. This dichotomous effect underscores the gene’s direct influence on tumor cell dynamics and implicates C1orf122 as a pivotal modulator of hepatocarcinogenesis.
At the molecular level, the study illuminates how C1orf122 intricately manipulates apoptotic pathways, tipping the balance in favor of cellular survival and tumor progression. Overexpression of C1orf122 engenders a notable downregulation of pro-apoptotic factors such as Bax and cleaved caspase-3, concurrently elevating the levels of anti-apoptotic proteins including total Bcl-2. This orchestrated suppression of programmed cell death provides tumor cells with a survival advantage, effectively subverting intrinsic cellular safeguards that would otherwise limit unchecked proliferation.
In addition to apoptosis evasion, C1orf122 exerts transformative effects on epithelial-to-mesenchymal transition (EMT), a fundamental biological process implicated in tumor invasiveness and metastatic dissemination. The research documents significant upregulation of canonical EMT markers—N-Cadherin, Vimentin, Slug, and Twist1—in response to heightened C1orf122 expression. Such molecular remodeling fosters enhanced migratory and invasive potential of HCC cells, thereby facilitating disease progression and metastasis, which are hallmarks of advanced liver cancer.
Crucially, the study uncovers the signaling cascade through which C1orf122 operationalizes its oncogenic effects. C1orf122 physically interacts with serine/arginine-rich protein-specific kinase 1 (SRPK1), catalyzing its phosphorylation at the Thr601 residue—a modification expertly mediated by mechanistic target of rapamycin (mTOR) kinase activity. This phosphorylation event serves as a critical molecular switch that activates the downstream PI3K/AKT/GSK3β pathway, a well-established axis driving cellular growth, survival, and metabolism in malignant contexts. The aberrant activation of this signaling network orchestrated by C1orf122 thereby establishes a direct mechanistic link to hepatocellular carcinoma pathophysiology.
The implications of these findings are multifaceted and profound. Not only does C1orf122 emerge as a robust biomarker capable of stratifying patient prognosis with high fidelity, but its role as a key upstream regulator of oncogenic signaling pathways highlights it as a prime candidate for targeted therapeutic intervention. By disrupting the C1orf122-SRPK1-mTOR axis, novel treatment modalities may be devised to curtail HCC progression, improving clinical outcomes in a disease known for its resistance to conventional therapies.
Moreover, the study’s methodology is notable for its translational relevance. Utilizing in vivo models, including subcutaneous tumor implants in nude mice infected with sg-Control or sg-C1orf122 constructs, the researchers validate that silencing C1orf122 significantly impedes tumor growth in a physiologically relevant setting. This critical experimental corroboration elevates the translational potential of C1orf122-targeting strategies from theoretical to practical horizons.
Aside from its oncological insights, this research exemplifies the power of integrative bioinformatics combined with molecular biology, demonstrating how big data from repositories like TCGA can be seamlessly integrated with classical benchwork to generate insights with direct clinical ramifications. This approach sets a new benchmark for future studies aiming to dissect the molecular underpinnings of complex cancers.
In summary, C1orf122 is definitively characterized as an oncogene in hepatocellular carcinoma, driving tumor initiation and progression by modulating apoptosis, EMT, and activating the SRPK1-dependent PI3K/AKT/GSK3β signaling cascade. Its elevated expression serves not only as a prognostic indicator but also as a gateway to novel molecular therapies. These advances underscore the urgent need to incorporate C1orf122 status into clinical decision-making frameworks and support further drug development efforts targeting its associated pathways.
As the global burden of liver cancer continues to escalate, uncovering molecular culprits like C1orf122 offers a beacon of hope for improved diagnostics and effective treatments. The findings from these researchers provide a compelling narrative that bridges fundamental molecular biology with translational oncology, potentially reshaping clinical paradigms and offering new lifelines to patients afflicted by hepatocellular carcinoma.
Subject of Research: Hepatocellular carcinoma; molecular mechanisms of tumor progression; role of C1orf122 in cancer signaling pathways.
Article Title: Identifying C1orf122 as a potential HCC exacerbated biomarker dependently of SRPK1 regulates PI3K/AKT/GSK3β signaling pathway
References: Jing Cai, Li Rong, Runzhi Wang, Zaikuan Zhang, Haiming Sun, Juan Chen, Dunchu Weng, Xinyi Li, Xiaosong Feng, Peiyi Lin, Shengming Xu, Zhihong Jiang, Yajun Xie, Qin Zhou. Genes & Diseases. DOI: 10.1016/j.gendis.2025.101721
Image Credits: Jing Cai, Li Rong, Runzhi Wang, Zaikuan Zhang, Haiming Sun, Juan Chen, Dunchu Weng, Xinyi Li, Xiaosong Feng, Peiyi Lin, Shengming Xu, Zhihong Jiang, Yajun Xie, Qin Zhou
Keywords: Hepatocellular carcinoma, C1orf122, SRPK1, PI3K/AKT/GSK3β pathway, tumor progression, apoptosis inhibition, epithelial-to-mesenchymal transition, mTOR kinase, biomarker, targeted therapy