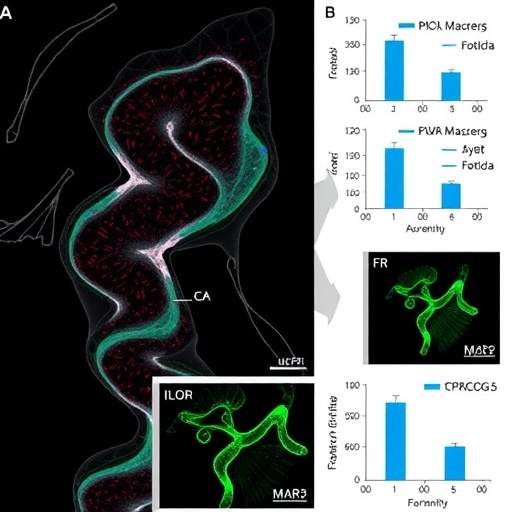
In a groundbreaking study published in Biochemical Genetics, researchers have unveiled a significant contributor to the complex mechanisms behind ovarian cancer—circCOG5. This circular RNA has emerged as a vital player in the regulation of ferroptosis, a form of regulated cell death characterized by the accumulation of lipid peroxides to lethal levels. As the intricacies of cancer metabolism and cell death pathways continue to be elucidated, circCOG5 stands out as a promising target for therapeutic intervention.
The treatment landscape for ovarian cancer, which remains one of the deadliest gynecological malignancies, is fraught with challenges, primarily due to chemoresistance and late-stage diagnoses. As scientists explore molecular targets that can enhance the efficacy of existing treatment modalities, the role of circRNAs has garnered considerable attention. The study by Guo and colleagues presents robust evidence that circCOG5 not only influences cell survival but also interacts with key regulatory pathways that control ferroptosis.
Ferroptosis, distinct from apoptosis and necrosis, plays an essential role in various diseases, including cancer. In the context of ovarian cancer, the induction of ferroptosis can suppress tumor growth by triggering a specific type of cell death sensitive to iron levels and lipid peroxidation. By investigating the interplay between circCOG5 and miR-532-3p, the researchers have illuminated a pathway that could potentially be exploited for therapeutic gain. Their findings suggest that circCOG5 acts as a sponge for miR-532-3p, thus alleviating the repression of LPCAT3, a key enzyme implicated in lipid metabolism.
Through experimental techniques including qRT-PCR, Western blotting, and functional assays, this study meticulously delineates the molecular interactions at play. The downregulation of circCOG5 was found to correlate with enhanced levels of miR-532-3p, which in turn led to decreased LPCAT3 expression, promoting a ferroptotic phenotype in ovarian cancer cells. This cascade of events highlights the delicate balance between circRNA expression and the miRNA network that governs cell fate.
The implications of the findings are profound. By contributing to our understanding of the molecular underpinnings of ferroptosis in ovarian cancer, circCOG5 could serve as a therapeutic target. This opens the door to the development of novel strategies aimed at enhancing ferroptotic cell death in ovarian tumors, potentially leading to more effective treatment regimens. The data suggest that manipulating the expression levels of circCOG5 may alter the susceptibility of cancer cells to ferroptosis-inducing agents, providing a dual approach to therapy by both sensitizing tumors to existing drugs and inducing a more aggressive cell death pathway.
Moreover, the ability of circCOG5 to modulate iron metabolism and lipid peroxidation underlies the necessity for further investigation into the regulatory networks involved. Understanding how circCOG5 interacts with other cellular components could unveil additional avenues for intervention. The intricate relationship between circular RNAs, miRNAs, and target genes presents a remarkable web of interactions that can either promote or inhibit cancer progression, warranting continued exploration.
This study serves as a pivotal reference for subsequent research aimed at pinpointing additional circRNAs that may fulfill similar roles in cancer biology. As the scientific community rallies to decipher the complexities of circRNAs and their contributions to oncogenesis and tumor microenvironments, the hope is that novel therapeutic strategies and biomarkers can be developed to improve outcomes for patients suffering from ovarian cancer.
In conclusion, the research spearheaded by Guo and colleagues lays the groundwork for a new chapter in the understanding of ovarian cancer biology and highlights the potential of circCOG5 as a therapeutic target. As we inch closer to personalized medicine, focusing on specific molecular signatures that govern tumor behavior can usher in a new era of targeted therapies. The quest for knowledge continues, but with discoveries such as these, the prospects are promising for creating more effective treatments that could significantly alter the trajectory of ovarian cancer management in the years to come.
While circRNA research is still in its nascent stages compared to linear RNA studies, the findings underscore the excitement and urgency behind expanding our understanding of this category of non-coding RNAs. Future investigations will undoubtedly refine these insights, bringing forth innovative therapeutic modalities that can surmount the challenges posed by ovarian cancer. As the battle against this malignancy progresses, circCOG5’s contributions to ferroptosis regulation may play a crucial role in redefining how we approach treatment and care for patients.
The journey of decoding the role of circRNAs in cancer is ongoing, but the significance of Guo et al.’s work cannot be understated. Their research not only enriches the current literature but also sets a precedent for future exploration in the field of cancer therapeutics. As we unveil the mechanisms that drive the death of cancer cells, we move closer to comprehending how to leverage these processes against one of the most challenging diseases we face today.
Subject of Research: Role of circCOG5 in Ovarian Cancer and Ferroptosis Regulation
Article Title: The Role and Mechanism of CircCOG5 in Regulating Ferroptosis in Ovarian Cancer Cells by Targeting miR-532-3p/LPCAT3
Article References:
Guo, Y., Wei, M., Fan, J. et al. The Role and Mechanism of CircCOG5 in Regulating Ferroptosis in Ovarian Cancer Cells by Targeting miR-532-3p/LPCAT3.
Biochem Genet (2025). https://doi.org/10.1007/s10528-025-11183-3
Image Credits: AI Generated
DOI: 10.1007/s10528-025-11183-3
Keywords: circRNA, circCOG5, ferroptosis, ovarian cancer, miR-532-3p, LPCAT3, targeted therapy, cancer biology.