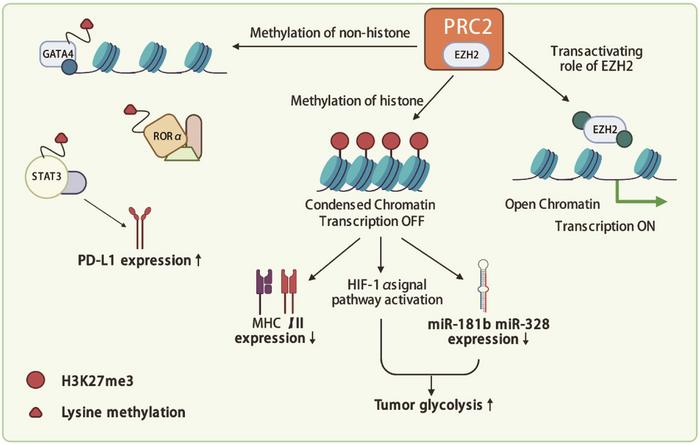
Enhancer of zeste homolog 2 (EZH2) is a catalytic subunit of the Polycomb-repressive complex 2 (PRC2), which plays a crucial role in transcriptional repression through the methylation of histone H3 on lysine 27 (H3K27me3). This epigenetic modification leads to chromatin compaction and gene silencing. EZH2 is frequently overexpressed in a variety of cancers, including head and neck, breast, prostate, bladder, colorectal, lung, pancreatic, melanoma, and lymphoma. Mutations in the EZH2 gene are also prevalent in several hematological malignancies, such as B-lymphomas and follicular lymphomas. The dual role of EZH2 as both a tumor suppressor and oncogene depending on the cancer type underscores its significance in cancer biology and therapy.

Credit: Wei Cao, Cheng-Zhong Lin, Xiao-Hu Lin, Wen-Kai Zhou
Enhancer of zeste homolog 2 (EZH2) is a catalytic subunit of the Polycomb-repressive complex 2 (PRC2), which plays a crucial role in transcriptional repression through the methylation of histone H3 on lysine 27 (H3K27me3). This epigenetic modification leads to chromatin compaction and gene silencing. EZH2 is frequently overexpressed in a variety of cancers, including head and neck, breast, prostate, bladder, colorectal, lung, pancreatic, melanoma, and lymphoma. Mutations in the EZH2 gene are also prevalent in several hematological malignancies, such as B-lymphomas and follicular lymphomas. The dual role of EZH2 as both a tumor suppressor and oncogene depending on the cancer type underscores its significance in cancer biology and therapy.
Mechanisms of EZH2 in Cancer
EZH2 influences cancer progression through various mechanisms, including promoting cell proliferation, inhibiting apoptosis, and enhancing metastatic potential. It achieves these effects by repressing tumor suppressor genes and modulating signaling pathways that control cell growth and survival. In addition to its role in tumor cells, EZH2 affects the tumor microenvironment, particularly immune cells, contributing to an immunosuppressive milieu that favors tumor evasion from immune surveillance.
EZH2 and the Tumor Microenvironment
Recent studies have highlighted the pleiotropic effects of EZH2 on both tumor and immune cells. EZH2 modulates the tumor immune microenvironment by affecting the function of various immune cells, including T cells, dendritic cells, and macrophages. This modulation can either promote or inhibit tumor growth, depending on the context. For example, EZH2 inhibition has been shown to increase the expression of immune checkpoint molecules like PD-1, thereby enhancing the efficacy of immunotherapies.
EZH2 Inhibitors in Cancer Therapy
The development of EZH2 inhibitors (EZH2i) represents a promising therapeutic strategy. Tazemetostat, an EZH2i, has already received FDA approval for the treatment of follicular lymphoma and epithelioid sarcoma. Preclinical studies and clinical trials are exploring the potential of combining EZH2 inhibitors with other therapeutic modalities, such as immune checkpoint blockade (ICB) and chimeric antigen receptor T-cell (CAR-T) therapy. These combinations aim to overcome resistance to single-agent therapies and enhance antitumor immune responses.
Combining EZH2 Inhibitors with Immunotherapy
Combining EZH2 inhibitors with immunotherapies has shown synergistic effects in preclinical models. For instance, the combination of EZH2 inhibitors with PD-1 blockers has demonstrated increased macrophage toxicity and enhanced immunotherapy efficacy in malignant pleural mesothelioma. Similarly, combining EZH2 inhibition with CAR-T therapy has improved therapeutic outcomes by boosting the expression of tumor-associated antigens in cancers like Ewing sarcoma. These findings suggest that EZH2 inhibitors can potentiate the effects of various immunotherapeutic approaches, offering new avenues for treating resistant and refractory cancers.
Future Perspectives
Despite the advances in tumor immunotherapy, challenges remain in achieving durable responses and overcoming resistance. The heterogeneous nature of tumors and the complexity of the tumor immune microenvironment necessitate a deeper understanding of the interplay between EZH2 and immune cells. Future research should focus on elucidating the specific mechanisms by which EZH2 modulates the immune microenvironment and identifying biomarkers to predict responses to combination therapies. Moreover, developing targeted and low-toxicity EZH2 inhibitors, possibly through nanotechnology and precision medicine approaches, holds promise for enhancing the efficacy and safety of cancer immunotherapy.
Conclusions
EZH2 plays a multifaceted role in cancer biology, influencing both tumor and immune cells. Its inhibition offers a promising strategy to enhance the efficacy of existing immunotherapies and overcome therapeutic resistance. Ongoing research and clinical trials will continue to shed light on the optimal use of EZH2 inhibitors in combination with other treatments, paving the way for more effective and personalized cancer therapies.
Full text
The study was recently published in the Cancer Screening and Prevention.
Cancer Screening and Prevention (CSP) publishes high-quality research and review articles related to cancer screening and prevention. It aims to provide a platform for studies that develop innovative and creative strategies and precise models for screening, early detection, and prevention of various cancers. Studies on the integration of precision cancer prevention multiomics where cancer screening, early detection and prevention regimens can precisely reflect the risk of cancer from dissected genomic and environmental parameters are particularly welcome.
Follow us on X: @xiahepublishing
Follow us on LinkedIn: Xia & He Publishing Inc.
Journal
Cancer Screening and Prevention
Article Title
The Heightened Importance of EZH2 in Cancer Immunotherapy
Article Publication Date
20-Jun-2023