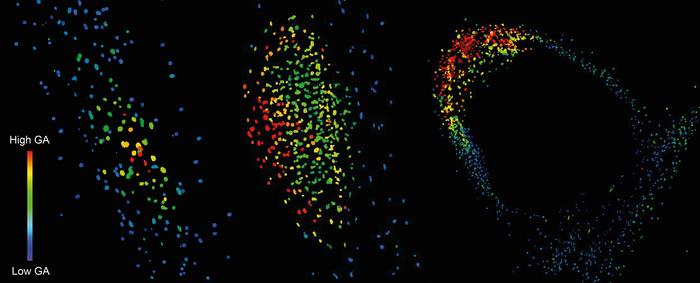
Researchers at the University of Cambridge demonstrate that the plant hormone gibberellin (GA) is essential for the formation and maturation of nitrogen-fixing root nodules in legumes and can also increase nodule size.

Credit: Colleen Drapek, Sainsbury Laboratory, University of Cambridge
Researchers at the University of Cambridge demonstrate that the plant hormone gibberellin (GA) is essential for the formation and maturation of nitrogen-fixing root nodules in legumes and can also increase nodule size.
They identify the specific times and location where GA governs the initiation, growth and function of nodules. These findings help to reconcile conflicting reports suggesting GA both inhibited and was required for nodulation by pinpointing the zones where GA is essential.
Cereal crops like wheat, maize and rice are nitrogen-hungry crops and depend heavily on synthetic fertilisers to meet their nitrogen needs. However, synthetic nitrogen fertilisers require an enormous amount of energy to manufacture, are expensive for farmers and cause negative environmental impacts like water pollution.
Unlike cereals, legumes, like peas, beans and pulses, can obtain their own nitrogen through a natural symbiotic relationship with nitrogen-fixing bacteria, forming lateral root-derived organs called nodules. This nitrogen-fixing ability also leads to higher protein content in legume crops, making them more nutritious for human consumption.
However, legume crops stop producing root nodules when the soil has relatively high concentrations of nitrogen and as a result potentially miss out on producing higher yields.
Scientists around the world are working on how to both boost legume yields and transfer nitrogen-fixing abilities from legumes to cereals, but this involves unravelling and understanding the complex genetic and biochemical pathways involved in nodule formation and nitrogen fixation.
In research published in The Plant Cell, Dr Alexander Jones’ research group at the Sainsbury Laboratory Cambridge University (SLCU) and Professor Giles Oldroyd’s group at the Crop Science Centre have made a major step towards this goal by revealing the GA dynamics that govern the development, morphology and function of nitrogen-fixing root nodules. Dr Jones said: “There were some confusing and conflicting reports about the function of GA in nodule symbiosis. Experiments showed that adding GA reduces nodulation and removing GA increases nodulation in legumes like Medicago truncatula, which suggests GA is antagonistic towards nodulation. But there is also a legume mutant in peas that produces less GA and has fewer nodules, which suggests that GA is somehow required for nodulation.”
“These conflicting results suggest there is probably something going on with spatial-temporal GA patterning. For example, there may be specific places where GA needs to be and some places where it needs to be absent. Or that the precise concentration of GA is important.”
Using the highly sensitive next-generation biosensor nlsGIBBERELLIN PERCEPTION SENSOR 2 (GPS2) developed in the Jones Group, Dr Colleen Drapek was able to visualise exactly where and when GA was present and in what relative concentrations it occurred. She found GA accumulated in the nodule primordium (the zone in the root cortex where cells start dividing in the early stages of nodule formation) in Medicago infected with rhizobium bacteria. Dr Drapek said: “Right at the beginning of nodule formation you start to see an accumulation of GA in the nodule primordia, but very little GA anywhere else in the root. As the root nodule further develops, you see GA accumulating at quite high concentrations and remaining at high levels in the mature nodule.”
Dr Drapek used GA and symbiotic Medicago mutants to further test what GA was doing by taregtting overexpression of enzymes that break GA down or synthesise GA. The result for the former was that no nodules formed and the latter was larger nodules. “This shows GA is very important for nodules, but that its function is specific to zones where the nodule is being initiated and not surrounding areas. We know that low GA is good for the initial rhizobium infection of the roots, but then later you need GA to be present for the nodulation process to proceed and for nodules to mature.”
In earlier research in the Oldroyd Group at SLCU undertaken by Dr Katharina Schiessl, it was shown that there is an overlap in the developmental programme that plants use to form lateral roots and nitrogen-fixing nodules. Professor Oldroyd said: “These latest findings show that GA accumulation in the root is unique to nodule development and likely therefore a critical switch for nodule-specific development. These are essential insights for us in attempting to transfer nitrogen-fixation to other crops such as cassava and cereals.”
Reference
Colleen Drapek, Annalisa Rizza, Nadiatul Radzman-Mohd, Katharina Schiessl, Fabio Dos Santos Barbosa, Jiangqi Wen, Giles E.D. Oldroyd, Alexander M. Jones (2024) GA dynamics governing nodulation revealed using GIBBERELLIN PERCEPTION SENSOR 2 in Medicago truncatula lateral organs. The Plant Cell.
Funding
This research was supported by the Gatsby Charitable Trust, EMBO, Marie Skłodowska-Curie Actions, Bill & Melinda Gates Foundation and the UK Government Foreign, Commonwealth and Development Office.
Journal
The Plant Cell
Method of Research
Experimental study
Subject of Research
Cells
Article Title
GA dynamics governing nodulation revealed using GIBBERELLIN PERCEPTION SENSOR 2 in Medicago truncatula lateral organs
Article Publication Date
23-Jul-2024