In the Earth’s subduction zones, water is transported into the deep mantle by nominally anhydrous minerals (NAMs) and water-bearing minerals in oceanic plates that react with seawater. Therefore, determination of the stability field and water content of water-bearing minerals is very important for understanding the water cycle processes in the Earth’s deep interior. SiO2 minerals are universally contained in the crust that makes up the Earth’s surface (continental and oceanic crust). Quartz is stable at the Earth’s surface, whereas stishovite is stable in the Earth’s mantle transition zone and lower mantle. Recent studies have shown that SiO2 stishovite retains large amounts of water (>1 wt%), and is thought to be a major water carrier in the lower mantle. It has been observed that, in water-saturated systems, the unit-cell volume of stishovite expands excessively with water dissolution (excess volume). However, the temperature and pressure conditions under which excessive volume expansion is observed in stishovite differ in previous studies due to the inability to carry out observations at well-controlled high-pressures and high temperatures under water-saturated conditions.

Credit: Goru Takaichi, Ehime University
In the Earth’s subduction zones, water is transported into the deep mantle by nominally anhydrous minerals (NAMs) and water-bearing minerals in oceanic plates that react with seawater. Therefore, determination of the stability field and water content of water-bearing minerals is very important for understanding the water cycle processes in the Earth’s deep interior. SiO2 minerals are universally contained in the crust that makes up the Earth’s surface (continental and oceanic crust). Quartz is stable at the Earth’s surface, whereas stishovite is stable in the Earth’s mantle transition zone and lower mantle. Recent studies have shown that SiO2 stishovite retains large amounts of water (>1 wt%), and is thought to be a major water carrier in the lower mantle. It has been observed that, in water-saturated systems, the unit-cell volume of stishovite expands excessively with water dissolution (excess volume). However, the temperature and pressure conditions under which excessive volume expansion is observed in stishovite differ in previous studies due to the inability to carry out observations at well-controlled high-pressures and high temperatures under water-saturated conditions.
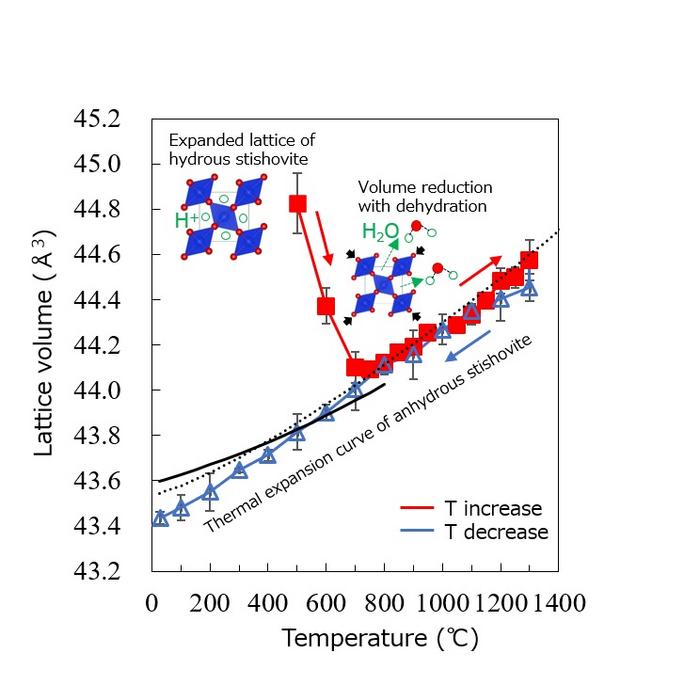
We developed a new technique for in situ X-ray observation in a water-saturated system at high-pressures and high-temperatures using a multi-anvil apparatus, and investigated changes in the unit-cell volume of SiO2 stishovite at 10-30 GPa and up to 1300°C. We found that the unit-cell volume of SiO2 stishovite was significantly larger than that of anhydrous stishovite only just after the first crystallization. The experimental results showed that the maximum volume expansion was 3.8%, and the excess volume decreased rapidly with increasing temperature and time, and the unit-cell volume was almost equal to the anhydrous value whenabove 700℃. Furthermore, no excess volume was observed during a subsequent temperature decrease. Thus, the dissolution of water into SiO2 stishovite may be a metastable phenomenon, and it is unlikely that hydrous SiO2 stishovite is a stable phase or an important water carrier, at least at the top of the lower mantle.
Journal
Earth and Planetary Science Letters