Radiation therapy has long been a cornerstone in the treatment of various malignancies, leading to considerable advancements in oncology. However, despite its efficacy in targeting cancer cells, radiation can also inflict damage on healthy tissues. Recent research spearheaded by Boncompagni et al. delves into a specific mechanism that may underlie radiation-induced cardiac injury—the role of the NLRP3 inflammasome, a crucial player in immune responses and inflammation. The findings suggest that by targeting this inflammasome, it may be possible to mitigate the cardiovascular complications that often arise following radiation treatment.
In the intricate dance of therapeutic interventions, radiation therapy excels in obliterating malignant cells. This highly targeted approach is, however, not without its adversities. Among the most concerning side effects is radiation-induced cardiac injury. As cancer survivors are living longer due to improved therapies, the long-term outcomes of such injuries are emerging as critical issues in survivorship care. The study by Boncompagni and colleagues provides key insights into the pathophysiological mechanisms at play, especially highlighting the NLRP3 inflammasome’s role in this phenomenon.
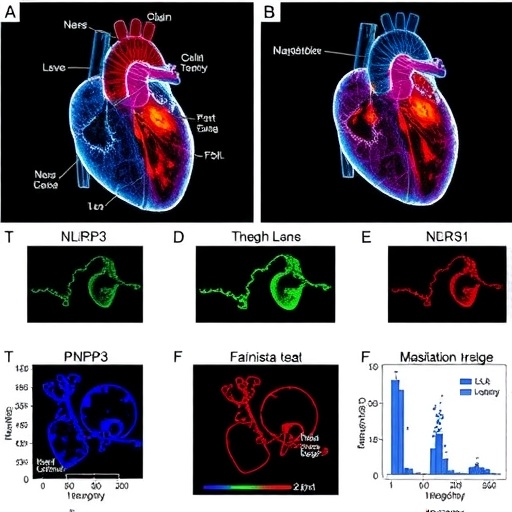
The NLRP3 inflammasome acts as a signaling hub that is activated during various forms of cellular stress. Its activation results in the processing and release of pro-inflammatory cytokines, particularly IL-1β and IL-18, which exacerbate inflammatory responses. In the context of radiation exposure, understanding the activation pathways of the NLRP3 inflammasome could illuminate why some patients experience severe cardiac toxicity while others do not. Boncompagni et al. meticulously detail how radiation can lead to the dysregulation of this inflammasome, initiating a cascade that harms cardiac cells and tissue.
Moreover, the research underscores the connection between inflammation and tissue damage in the heart. Inflammation, while a natural response to injury or infection, becomes detrimental when it is persistent or uncontrolled. The study emphasizes how radiation exacerbates this inflammatory state, leading not only to immediate cellular damage but also contributing to long-term cardiac remodeling and dysfunction. This insight positions the NLRP3 inflammasome as a potential therapeutic target for reducing the incidence of cardiac injury in patients undergoing radiotherapy.
The authors employed an array of experimental models to elucidate the role of the NLRP3 inflammasome in radiation-induced cardiac injury. By exposing cardiac tissue models to radiation and subsequently measuring inflammasome activation markers, they provided compelling evidence that supports the hypothesis. These experiments aim to establish a molecular link between radiation exposure and the inflammatory responses that lead to cardiac sequelae.
The implications of this work extend beyond the bench as they prompt the re-evaluation of patient management strategies. By integrating therapies aimed at modulating inflammasome activity, oncologists could potentially offer a dual approach: effectively treating cancer while protecting heart health. This integrated treatment model could enhance the quality of life for cancer survivors who previously experienced the burden of cardiovascular issues arising from radiation therapy.
Additionally, the research paves the way for future studies exploring specific inhibitors of the NLRP3 inflammasome. The development of targeted therapeutics could provide oncologists with the necessary tools to simultaneously manage cancer and alleviate inflammatory side effects. As the medical community moves towards personalized medicine, this research strongly advocates for considering individual inflammatory profiles when designing therapy regimens.
Notably, there remain many unknowns regarding the specific pathways through which radiation induces NLRP3 inflammasome activation. Understanding how various doses and fractionation schedules impact inflammasome signaling is imperative for designing optimal treatment plans. Furthermore, examining the genetic predisposition of individuals to inflammasome hyperactivation could yield invaluable insights into personalized treatment strategies.
As the body of research surrounding the NLRP3 inflammasome continues to grow, the potential for translational applications becomes increasingly evident. While Boncompagni et al.’s findings represent a significant leap forward in understanding radiation-induced cardiac injury, they also highlight the necessity of more extensive clinical trials to validate the preclinical observations. Future investigations will need to assess the safety and efficacy of interventions targeting the inflammasome in the context of radiotherapy.
The study’s findings underscore an urgent need for multidisciplinary collaboration between oncologists, cardiologists, and researchers to bridge the gap between basic science and clinical implications. The nexus of radiation therapy, inflammation, and heart health beckons a holistic approach, ensuring that cancer care encompasses survivorship and long-term wellness. This integrative strategy must be reflected in future clinical guidelines, addressing both cancer elimination and the preservation of cardiovascular health.
Looking ahead, it is crucial that researchers continue to unravel the complexities of the NLRP3 inflammasome’s role in not only cardiac injury but also other radiation-induced complications. As we advance towards a more refined understanding of these mechanisms, the hope is to ultimately revolutionize care for cancer patients, ensuring their journey through treatment is met with comprehensive support tailored to sustain their health.
In conclusion, the work of Boncompagni and colleagues not only sheds light on a pivotal aspect of radiation-induced damage but also reinforces the notion that our understanding of cancer therapy must evolve. By recognizing the interplay between inflammatory pathways and treatment-related side effects, the scientific community can better serve the needs of patients, paving the way for a future where cancer treatment does not come at the cost of vitality and well-being.
As we await the full ramifications of these exciting findings, the promise they hold is immense. By harnessing the power of targeted therapies against the NLRP3 inflammasome, we may well see a revolution not just in cancer survival rates, but in the quality of life for an ever-growing population of cancer survivors.
Subject of Research: Radiation-induced cardiac injury and the role of NLRP3 inflammasome.
Article Title: Radiation meets inflammation: NLRP3 inflammasome at the core of radiation-induced cardiac injury.
Article References:
Boncompagni, C., Giacovazzi, S., Perrone, M. et al. Radiation meets inflammation: NLRP3 inflammasome at the core of radiation-induced cardiac injury. J Transl Med 23, 1330 (2025). https://doi.org/10.1186/s12967-025-07377-3
Image Credits: AI Generated
DOI: https://doi.org/10.1186/s12967-025-07377-3
Keywords: Radiation therapy, cardiac injury, NLRP3 inflammasome, inflammation, cancer treatment, cytokines, therapeutic target, survivorship care.