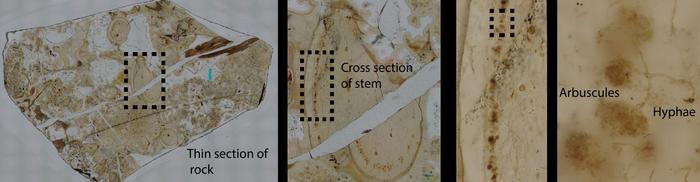
In a groundbreaking study that pushes the frontiers of paleobiology and microscopy, an international consortium of researchers from the Natural History Museum in London, the Sainsbury Laboratory at Cambridge University, and the Cambridge Graphene Centre has identified a new species of ancient symbiotic fungus embedded within a 407-million-year-old plant fossil from the Windyfield Chert in Scotland. This discovery, published in the esteemed journal New Phytologist, illuminates one of the earliest known plant–fungus partnerships, fundamentally advancing our understanding of early terrestrial ecosystems and their complex biological interactions.
Harnessing the power of advanced imaging techniques, including confocal fluorescence lifetime imaging microscopy (FLIM) combined with Raman spectroscopy, the research team unveiled the exquisite three-dimensional preservation of fungal structures nestled within the primitive land plant Aglaophyton majus. This marks the first time such detailed morphological and chemical information has been retrieved from a fossil this ancient, heralding a significant leap in the methodological approach to paleontological analysis. The novel fungal species, named Rugososporomyces lavoisierae, is the second arbuscular mycorrhizal fungus identified in association with Aglaophyton majus, underscoring the complexity of early mycorrhizal symbioses.
The Windyfield Chert fossil, housed at the National Museum of Scotland, provided a pristine window into early land colonization by plants and their microbial partners. The newly described fungus displays intricate arbuscules, highly branched intracellular structures instrumental in nutrient exchange between fungi and plants. These arbuscular formations typify a mutualistic relationship wherein the fungal partner enhances mineral nutrient uptake—particularly phosphorus—while deriving photosynthetically produced sugars from its host. Crucially, the presence of these structures within the fossilized tissues confirms that the interaction was symbiotic rather than parasitic or saprophytic, affirming the ecological sophistication of early terrestrial life forms.
Dr. Christine Strullu-Derrien, a leading paleobiologist and co-lead of the study, emphasized the rarity and significance of mycorrhizal evidence in the fossil record. “Given the scarcity of early plant-fungal symbioses in ancient deposits, our findings reveal an intricate ecological alliance that presumably facilitated plant adaptation to the terrestrial environment. This partnership was pivotal in surmounting nutrient limitations faced by pioneering land plants lacking true roots.” Her remarks highlight how such symbiotic relationships were critical evolutionary innovations that enabled plants to thrive beyond aquatic habitats.
The study’s interdisciplinary nature was integral to its success. Experts combined classical paleobotanical methods, such as polished thin section microscopy under high-resolution Keyence microscopes, with state-of-the-art spectral imaging techniques. Fluorescence lifetime imaging captured the unique optical signatures of organic compounds preserved as carbonaceous residues, while Raman spectroscopy provided complementary molecular fingerprints. These combined modalities allowed researchers to distinguish fossilized fungal hyphae and arbuscules from surrounding plant cellular material with unprecedented precision, offering a novel way to interrogate ancient microstructures chemically as well as morphologically.
Dr. Raymond Wightman, who spearheaded the FLIM imaging, stated, “The synergy between fluorescence lifetime measurements and Raman spectroscopic data enables us to create a multidimensional chemical map of fossil tissues. This approach transcends traditional paleontological techniques by revealing biochemical heterogeneity frozen in time, thereby uncovering cryptic life signatures long after DNA degrades.” His insights underscore the technological innovation underpinning this discovery and its potential to revolutionize the field.
Co-author Dr. Paul Kenrick, an expert in fossil plants, further contextualized the significance of this ancient symbiosis by comparing it with associations observed in modern non-vascular plants like liverworts and hornworts. These extant relatives also engage in arbuscular mycorrhizal partnerships despite lacking true roots, suggesting a deep evolutionary continuity. Such insights refine our understanding of how early plants harnessed microbial collaborations to colonize and stabilize terrestrial habitats—a key chapter in the narrative of life’s expansion onto land.
Beyond evolutionary biology, the implications of this research extend into methodological innovations poised to influence paleobiology broadly. The team anticipates that this advanced imaging and spectroscopic toolbox can be applied to diverse fossilized organisms — including early arthropods and other plants — resolving fine ultrastructural differences invisible through conventional light microscopy. This chemical and optical “fingerprinting” offers a remarkable capability to discriminate between morphologically similar fossils, opening a new era in the identification and classification of ancient life forms.
Professor Sebastian Schornack, co-leader of the project, highlighted these future prospects, remarking, “By exploiting unique light signatures emitted by fossil materials, we gain a multidimensional perspective that redefines our ability to decode biological identity in deep time. This methodological breakthrough fosters a more nuanced reconstruction of evolutionary histories enriched by chemical data previously inaccessible in paleontological research.”
Looking forward, the research collective is poised to apply these imaging techniques extensively to other fossil assemblages from the Windyfield and neighboring Rhynie cherts. Such work aims to elucidate how early mycorrhizal symbioses diversified and how plants forged alliances with multiple fungal partners during the Devonian terrestrial revolution. This ongoing investigation promises not only to deepen our comprehension of ancient ecological networks but also to inform contemporary perspectives on plant-fungal interactions essential for ecosystem resilience.
This pioneering study exemplifies the power of interdisciplinary collaboration, merging paleontology, plant biology, biophysics, and materials science. The integration of expertise from microscopy specialists, geobiologists, and graphene researchers crafted an innovative pathway toward unearthing life’s ancient symbiotic legacies. As this technology matures, it stands to enrich the scientific toolkit capable of reconstructing life’s primordial chapters with breathtaking clarity and molecular detail.
In sum, the discovery of Rugososporomyces lavoisierae within a 407-million-year-old fossil enshrines a landmark achievement that bridges the gap between fossil morphology and chemistry. It validates the enduring nature of biological partnerships essential for life on land and augments our technological arsenal with sophisticated imaging modalities. Future applications promise a renaissance in fossil analysis, unveiling secrets of early life previously veiled by millions of years, heralding exciting paradigms in evolutionary biology and paleontological technique.
Subject of Research: Not applicable
Article Title: An arbuscular mycorrhiza from the 407-million-year-old Windyfield chert identified through advanced fluorescence and Raman imaging
News Publication Date: 12-Nov-2025
Web References:
- https://www.nhm.ac.uk/
- https://www.slcu.cam.ac.uk/
- https://www.graphene.cam.ac.uk/
- https://nph.onlinelibrary.wiley.com/doi/10.1111/nph.70655
References:
Christine Strullu-Derrien, Raymond Wightman, Liam McDonnell, Gareth Evans, Frédéric Fercoq, Paul Kenrick, Andrea Ferrari and Sebastian Schornack (2025) An arbuscular mycorrhiza from the 407-million-year-old Windyfield chert identified through advanced fluorescence and Raman imaging. New Phytologist. DOI: https://doi.org/10.1111/nph.70655
Image Credits: Gareth Evans
Keywords: Fossils, Paleoethnobotany, Plant fossils, Mycorrhizae, Symbiosis