In a groundbreaking preclinical study published in the journal Gastroenterology, researchers from Weill Cornell Medicine have unveiled a revolutionary approach to treating short bowel syndrome by repurposing the genetic mechanisms of the colon. This innovative technique utilizes the deletion of a specific gene, SATB2, to provoke a transformation in colon cells, allowing them to acquire properties akin to those of the small intestine. The implications of this work extend far beyond a mere academic endeavor; it presents a potential pathway for therapeutic interventions in a condition that currently poses significant challenges to patient health and quality of life.
Short bowel syndrome, a severe malabsorption disorder, arises when surgical removal of a considerable portion of the small intestine leaves patients with inadequate capacity to absorb nutrients. Common causes include inflammatory bowel disease, cancer resections, trauma, and congenital anomalies, resulting in many patients being reliant on intravenous nutrition for survival. As the principal site for nutrient absorption and digestion, the small intestine’s critical role in human physiology underscores the necessity of innovative treatment modalities for conditions that compromise its function.
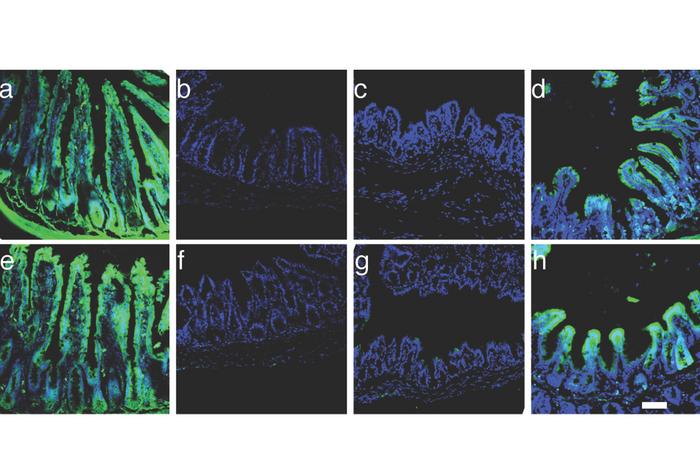
The pioneering study, led by Dr. Xiaofeng Steve Huang and his dedicated team, highlights the significant role of SATB2, which is known to maintain the identity of colon cells. Previous findings revealed that the absence of SATB2, whether in murine models or human colon cells, induces a phenotypic shift in those cells, prompting them to exhibit characteristics of ileal cells, specifically the lower section of the small intestine. This conversion could be harnessed therapeutically to restore nutrient absorption capacities in the colon, particularly for patients suffering from short bowel syndrome.
In the research, genetically modified mice lacking SATB2 demonstrated remarkable recovery. Notably, these mice not only regained their body weight but showed increased survival rates compared to control mice that retained the gene. The experimental mice achieved a survival rate of over 80% beyond 60 days, while the control group exhibited only a 10% survival rate. The observed transformations in the tissue architecture of the colon were striking, with the upper colon of SATB2-deficient mice beginning to resemble ileal tissue, indicating a potential for nutrient absorption comparable to that of the small intestine.
In a pivotal advancement, the researchers utilized organoids derived from human colon cells to further test their strategy. These small, 3D tissue-like structures accurately mimic the properties of actual human tissues, providing an invaluable platform for studying cellular transformations and interactions. Upon introducing an adenovirus-associated virus (AAV) of the gene editor, the organoids altered their genetic makeup—deleting SATB2 and thus acquiring ileal-like properties. Remarkably, these modified organoids not only survived but thrived when transplanted into mice, showcasing the viability of this approach for future therapeutic applications.
Dr. Huang and his colleagues are acutely aware of the ethical implications and the paramount importance of conducting further studies before proceeding to human trials. While the potential for a gene therapy based on these findings appears promising, extensive preclinical testing remains necessary to ensure safety and efficacy. The team is committed to advancing their understanding of how these genetic modifications can influence overall health, particularly for individuals facing the challenges of short bowel syndrome.
Parallel research into the genetic underpinnings of the gastrointestinal tract emphasizes the intricate balance maintained within this system. The large intestine, primarily responsible for water absorption, is structurally and functionally distinct from the small intestine. However, by tapping into the genetic regulatory circuits that steer cell identity, researchers open new avenues for reprogramming cellular behavior in an effort to ameliorate conditions induced by substantial intestinal loss.
The tragic loss of Dr. Qiao Zhou, a guiding figure in this research, has undoubtedly lent weight to the emotional and ethical considerations surrounding the publication of this work. His contributions to the understanding of SATB2’s functional implications in gastrointestinal biology have paved the way for this significant advancement in regenerative medicine.
Through this research, the authors aim not only to explore the feasibility of reprogramming colon cells for enhanced nutrient absorption but also to establish foundational insights into the interplay of genetic factors in gastrointestinal health. The acknowledgment of gene therapy’s potential in treating chronic conditions like short bowel syndrome marks a turning point in regenerative medicine, demonstrating that gene editing techniques could offer solutions to some of the most pressing medical challenges.
In conclusion, the findings from Weill Cornell Medicine present a compelling frontier in gastrointestinal research. As scientists continue to investigate and apply genetic engineering solutions, we may one day witness the transition of such groundbreaking techniques from the laboratory to the clinic, ushering in a new era of precision medicine that empowers patients with previously insurmountable conditions. It is this blend of innovative science and compassionate care that may one day transform the landscape of treatment options for individuals battling short bowel syndrome.
Subject of Research: Gene therapy targeting SATB2 for treating short bowel syndrome
Article Title: Remodeling the colon with ileal properties to treat short bowel syndrome
News Publication Date: 3-Apr-2025
Web References: [Not available]
References: [Not available]
Image Credits: Dr. Tao Liu
Keywords: Gene therapy, SATB2, short bowel syndrome, gastrointestinal system, nutrient absorption, organoids, regenerative medicine, adenovirus-associated virus, preclinical models, molecular biology, Weill Cornell Medicine.