Capacitors are crucial components in electronic devices such as smartphones and computers. They are made of dielectric materials that polarize on the application of the voltage. Currently, barium titanate (BaTiO₃) is the most widely used material for capacitors. Barium titanate belongs to the perovskite group of materials, where a titanium ion resides within an oxygen octahedral cage. The material exhibits displacive-type ferroelectric behavior, where the displacement of ions during the phase transition leads to the creation of a permanent dipole moment within the material.

Credit: Ayako Yamamoto from SIT, Japan
Capacitors are crucial components in electronic devices such as smartphones and computers. They are made of dielectric materials that polarize on the application of the voltage. Currently, barium titanate (BaTiO₃) is the most widely used material for capacitors. Barium titanate belongs to the perovskite group of materials, where a titanium ion resides within an oxygen octahedral cage. The material exhibits displacive-type ferroelectric behavior, where the displacement of ions during the phase transition leads to the creation of a permanent dipole moment within the material.
In a study published in the journal Dalton Transactions, 2024, 53, 7044 -7052 on April 1, 2024, researchers led by Professor Ayako Yamamoto from the Shibaura Institute of Technology, including master student Kimitoshi Murase have developed a displacement-type ferroelectric material with a high dielectric constant. The theoretical part was investigated by Dr. Hiroki Moriwake and his group from the Japan Fine Ceramics Center.
Employing a high-pressure method, researchers successfully incorporated sizable rubidium ions into perovskite-type compounds, resulting in the synthesis of rubidium niobate (RbNbO3). This compound, previously known for its challenging synthesis process, was effectively created through an innovative approach. RbNbO3 exhibits displacement ferroelectricity like BaTiO3, making it a promising candidate for capacitors and interest in synthesizing RbNbO3 dates back to the 1970s. However, investigations into its dielectric properties have only been conducted at low temperatures (below 27°C). This study sheds light on the crystal structure and phase transitions across a broad temperature range (–268 to +800°C), paving the way for further research and development.
“The high-pressure synthesis method has reported a variety of materials with perovskite-type structures, including superconductors and magnets. In this study, our focus was on combining niobates and alkali metals known for their high dielectric properties,” says Prof. Yamamoto.
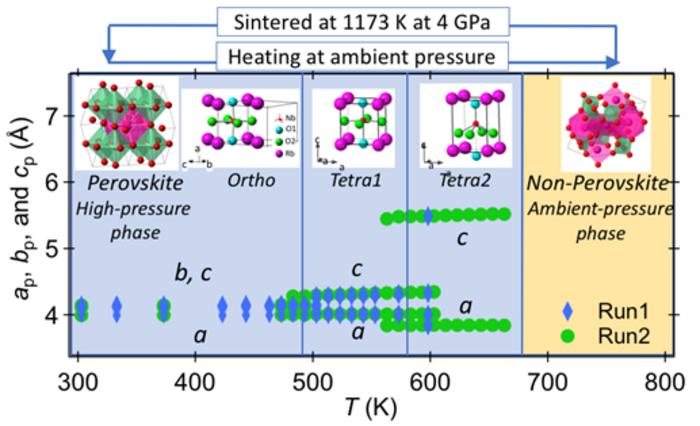
The researchers synthesized non-perovskite-type RbNbO3 by sintering a mixture of rubidium carbonate and niobium oxide at 1073 K (800°C), then subjected it to high pressures of 40,000 atmospheres at 1173 K (900°C)for 30 minutes. Under these high-pressure and high-temperature conditions, the rubidium niobate underwent a structural transformation from a complex triclinic phase at ambient pressure phase into a 26 % denser orthorhombic perovskite-type structure.
Using X-ray diffraction, the researchers investigated the crystal structure. Their analysis using a single crystal revealed that the crystal structure closely resembled that of potassium niobate (KNbO3) and exhibited similar distortions observed in BaTiO3, both well-known ferroelectric materials. However, they found that the orthorhombicity and displacement of niobium atoms in RbNbO3 exceeded those of KNbO3, indicating a higher degree of dielectric polarization due to phase transitions.
Furthermore, through powder X-ray diffraction, researchers identified four distinct phase transitions occurring across temperatures ranging from –268°C to +800°C. Below room temperature, RbNbO3 exists in an orthorhombic phase, which is the most stable configuration. As the temperature rises, it undergoes transitions: first to a tetragonal perovskite phase above 220°C, then into a more elongated tetragonal perovskite phase beyond 300°C. Finally, above 420°C, it reverts to a non-perovskite phase found under atmospheric conditions.
These observed phase transitions closely match predictions made through first-principles calculations. The researchers also calculated the dielectric polarization of different phases of RbNbO3. They found that the orthorhombic phase had a polarization of 0.33 C m−2, while the two tetragonal phases showed polarizations of 0.4 and 0.6 C m−2, respectively. These values are comparable to those of ferroelectric alkali metal niobates such as KNbO3 (0.32 C m−2), LiNbO3 (0.71 C m−2), and LiTaO3 (0.50 C m−2).
“The high-pressure phase obtained this time confirmed the presence of a polar structure from the observation of second harmonic generation of the same strength as potassium niobate, and a relatively high relative permittivity was also obtained. As for the dielectric constant, it is expected that values equal to or greater than those of potassium niobate can be obtained by increasing the sample density, as predicted from theoretical calculations,” says Prof. Yamamoto.
The researchers are planning further experiments to accurately measure the dielectric constant and demonstrate the high polarization of RbNbO3. The advantage of the high-pressure method lies in its ability to stabilize substances that do not exist under atmospheric pressure. Using the proposed method, larger alkali metal ions such as cesium could be incorporated into the perovskite structure, leading to ferroelectrics with desirable dielectric properties.
***
Reference
About Shibaura Institute of Technology (SIT), Japan
Shibaura Institute of Technology (SIT) is a private university with campuses in Tokyo and Saitama. Since the establishment of its predecessor, Tokyo Higher School of Industry and Commerce, in 1927, it has maintained “learning through practice” as its philosophy in the education of engineers. SIT was the only private science and engineering university selected for the Top Global University Project sponsored by the Ministry of Education, Culture, Sports, Science and Technology and will receive support from the ministry for 10 years starting from the 2014 academic year. Its motto, “Nurturing engineers who learn from society and contribute to society,” reflects its mission of fostering scientists and engineers who can contribute to the sustainable growth of the world by exposing their over 9,500 students to culturally diverse environments, where they learn to cope, collaborate, and relate with fellow students from around the world.
About Professor Ayako Yamamoto from SIT, Japan
Ayako Yamamoto is a Professor at the College of Engineering, Shibaura Institute of Technology. She heads the Yamamoto Laboratory, which focuses on developing new materials using high-pressure methods. Dr. Yamamoto has published approximately 191 papers spanning the fields of solid-state chemistry, superconductivity, crystal chemistry, and high-pressure synthesis. Additionally, she serves as a director at the Japan Physical Society and vice representative director of NPO STEM Career Path Project for Girls (GSTEM-CPP), Japan.
Journal
Dalton Transactions
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Crystal structure and properties of perovskite-type rubidium niobate, a high-pressure phase of RbNbO3
Article Publication Date
1-Apr-2024
COI Statement
There are no conflicts to declare.