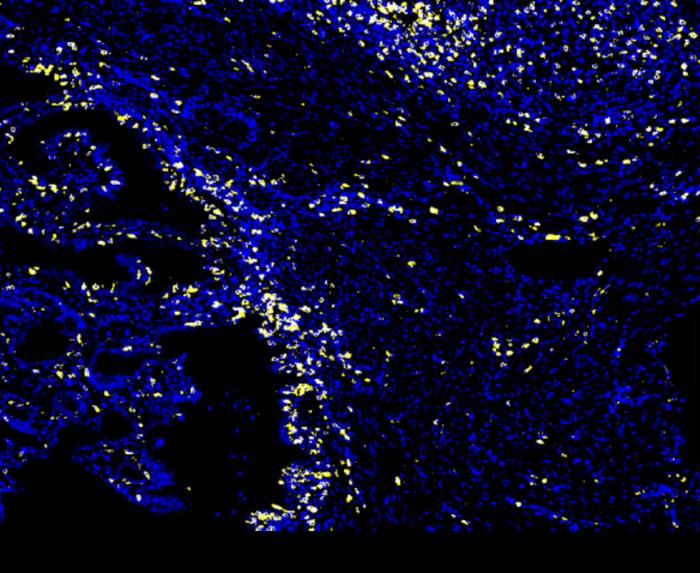
LA JOLLA (May 15, 2024)—Immunotherapy has revolutionized the way we treat cancer in recent years. Instead of targeting the tumor itself, immunotherapies work by directing patients’ immune systems to attack their tumors more effectively. This has been especially impactful in improving outcomes for certain difficult-to-treat cancers. Still, fewer than half of all cancer patients respond to current immunotherapies, creating an urgent need to identify biomarkers that can predict which patients are most likely to benefit.

Credit: Salk Institute
LA JOLLA (May 15, 2024)—Immunotherapy has revolutionized the way we treat cancer in recent years. Instead of targeting the tumor itself, immunotherapies work by directing patients’ immune systems to attack their tumors more effectively. This has been especially impactful in improving outcomes for certain difficult-to-treat cancers. Still, fewer than half of all cancer patients respond to current immunotherapies, creating an urgent need to identify biomarkers that can predict which patients are most likely to benefit.
Recently, scientists have noticed that patients whose tumors have a mutation in a gene called ARID1A are more likely to respond positively to immune checkpoint blockade, a type of immunotherapy that works by keeping cancer-fighting immune cells called T cells turned “on” when they’d otherwise be turned “off.” Since this ARID1A gene mutation is present in many cancers—including endometrial, ovarian, colon, gastric, liver, and pancreatic cancers—researchers at the Salk Institute wondered how it contributes to treatment sensitivity, and how clinicians can use this information to customize cancer treatments to each patient.
The new study, published in Cell on May 15, 2024, reveals that ARID1A mutation renders tumors sensitive to immunotherapy by inviting cancer-fighting immune cells into the tumor through an antiviral-like immune response. The researchers suggest this mutation and antiviral immune response could be used as a biomarker to better select patients for specific immunotherapies, like immune checkpoint blockade. The findings also encourage the development of drugs that target ARID1A and related proteins as a way of sensitizing other tumors to immunotherapy.
“This could really make a difference in patient outcomes from cancer treatment,” says Associate Professor Diana Hargreaves, senior author of the study. “These ARID1A mutation cancer patients are already having an immune response, so all we need to do is upregulate that response using immune checkpoint blockade to help them destroy their tumors from the inside.”
While it was reported that people with ARID1A mutations responded well to immune checkpoint blockade, the exact relationship between the two remained unclear. To elucidate the mechanism behind this, Salk scientists turned to mouse models of melanoma and colon cancer with either mutated ARID1A or functional ARID1A.
The team observed a powerful immune response in all animal models with mutated ARID1A tumors but not those with functional ARID1A tumors, supporting the idea that the ARID1A mutation was, indeed, driving the response. But how did this work on a molecular level?
“We found that ARID1A plays an important role in the nucleus of keeping DNA properly arranged,” says Matthew Maxwell, first author of the study and a graduate student in Hargreaves’ lab. “Without functional ARID1A, loose DNA can be excised and escape into the cytosol, which activates a coincidentally desirable antiviral immune response that can be further enhanced by immune checkpoint blockade.”
The ARID1A gene codes for a protein that helps regulate the shape of our DNA and maintain genome stability. When ARID1A is mutated, a microscopic chain of events analogous to a Rube Goldberg machine is set off in the cancer cell. First, the lack of functional ARID1A leads to escape of DNA into the cytosol. Next, the cytosolic DNA activates an antiviral alarm system—the cGAS-STING pathway—since our cells are adapted to flag any DNA in the cytosol as foreign to protect us against viral infections. Finally, the cGAS-STING pathway calls on the immune system to recruit T cells into the tumor and activates them into specialized cancer-killing T cells.
With each step relying on the last, this chain of events—ARID1A mutation, DNA escape, cGAS-STING alarm, T cell recruitment—results in more cancer-fighting T cells in the tumor. Immune checkpoint blockade can then be used to ensure these T cells stay “on,” supercharging them to defeat the cancer.
“Our findings provide a novel molecular mechanism by which ARID1A mutation can promote an anti-tumor immune response,” says Hargreaves. “What’s most exciting about these results is their translational potential. Not only can we use ARID1A mutations to help select patients for immune checkpoint blockade, but we now also see a mechanism by which drugs that inhibit ARID1A or its protein complex could be used to further enhance immunotherapy in other patients.”
By outlining the mechanism by which immune checkpoint blockade is more effective for ARID1A mutant cancers, the researchers have provided cause for clinicians to prioritize the immunotherapy for patients with mutated ARID1A. The findings are a major step in personalizing cancer treatment and inspiring novel therapies that target and inhibit ARID1A and its protein complex.
In the future, the Salk team hopes its findings can improve patient outcomes across the many cancer types associated with ARID1A mutations and is set to explore this clinical translation with collaborators at UC San Diego.
Other authors include Jawoon Yi, Shitian Li, Samuel Rivera, Jingting Yu, Mannix Burns, Helen McRae, Braden Stevenson, Josephine Ho, Kameneff Bojorquez Gastelum, Joshua Bell, Alexander Jones, Gerald Shadel, and Susan Kaech of Salk; Marianne Hom-Tedla and Katherine Coakley of Salk and UC San Diego; Ramez Eskander of UC San Diego; and Emily Dykhuizen of Purdue University.
The work was supported by the National Institutes of Health (NCI CCSG P30 014195, T32DK007541, R01 CA228211, R01 CA285867, R01 CA216101, R01 CA240909, R01 AI066232, R21 MH128678, S10-OD023689), National Science Foundation, Howard Hughes Medical Institute, Cancer Research Institute, Pew-Stewart Scholars for Cancer Research, American Cancer Society, and Padres Pedal the Cause.
About the Salk Institute for Biological Studies:
Unlocking the secrets of life itself is the driving force behind the Salk Institute. Our team of world-class, award-winning scientists pushes the boundaries of knowledge in areas such as neuroscience, cancer research, aging, immunobiology, plant biology, computational biology, and more. Founded by Jonas Salk, developer of the first safe and effective polio vaccine, the Institute is an independent, nonprofit research organization and architectural landmark: small by choice, intimate by nature, and fearless in the face of any challenge. Learn more at www.salk.edu.
Journal
Cell
Article Title
ARID1A suppresses R-loop mediated STING-Type I Interferon pathway activation of anti-tumor immunity
Article Publication Date
15-May-2024