Researchers at Kanazawa University report in eLife on deciphering the actin structure-dependent preferential cooperative binding of cofilin.

Credit: 2024, Ngo, Vu et al. eLife.
Researchers at Kanazawa University report in eLife on deciphering the actin structure-dependent preferential cooperative binding of cofilin.
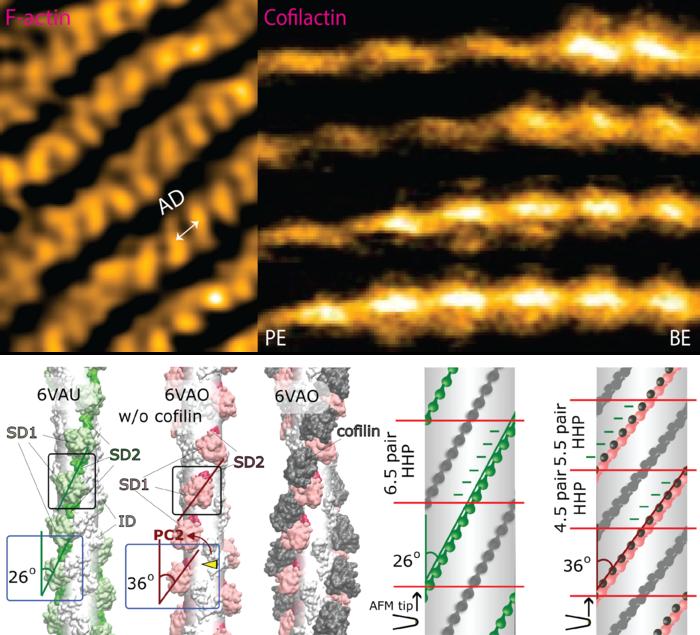
The actin filament is a double-stranded helical structure formed by intertwining two long-pitch helices, with the distance between crossover points, known as the half helical pitch (HHP), being about 36 nm. A canonical half helix consists of 13 actin protomers, or 6.5 protomer pairs, resulting in a mean axial distance (MAD) of 5.5 nm between two adjacent protomers along the same long-pitch strand. Cofilin is part of the actin-depolymerizing factor/cofilin (ADF/cofilin) family, is present in all eukaryotes. In mammals, there are two major isoforms: cofilin 1, found mainly in non-muscle tissues, and cofilin 2, found predominantly in muscle tissues. Cofilin is a crucial regulator of actin filament dynamics, especially in assembly and disassembly processes under nonequilibrium conditions. It facilitates the depolymerization and severing of actin filaments in a concentration-dependent manner and interacts with other actin-binding proteins either collaboratively or competitively in this role.
This study addresses several key questions to clarify the preferential cooperative binding and expansion of cofilin 1 clusters along actin filament: (i) Does the number of actin protomers per HHP differ between canonical actin filament and cofilin-undecorated twisted actin (C-actin-like) region? (ii) Does the MAD in these regions change over time, and how do these changes influence cofilin binding within the unbound actin regions containing ADP or ADP.Pi adjacent to cofilin clusters on the pointed-end (PE) and barbed-end (BE) sides?
Ngo and colleagues at WPI NanoLSI-Kanazawa University (Japan), Warwick University (United Kingdom), Hanoi University of Science and Technology (Vietnam), and Waseda University (Japan) leverage the high spatiotemporal resolution of high-speed atomic force microscopy (HS-AFM) to observe dynamic structural changes in actin filaments caused by cofilin binding. They also experimentally demonstrate the inherent variability in the twist conformations of bare actin filaments. Their findings reveal that the helical twists and dynamics of actin filaments are variable and irregular both in vitro and in vivo, challenging the traditional view from Cryo-Electron Microscopy and X-ray diffraction that these parameters are static. By integrating HS-AFM with Principal Component Analysis (PCA), the study elucidates the structure-dependent preferential cooperative binding of cofilin toward the PE side of the actin filament. They provide experimental evidence supporting the “proof of principle” that the flexible and specific helical twists of actin filaments regulate the functions of actin binding proteins.
Ngo and colleagues emphasize the importance of considering the structural dynamics and heterogeneity of filamentous actin in various nucleotide states, both with and without cofilin, over time. This significant study enhances our understanding of the dynamic and polymorphic nature of actin filaments, which are crucial for a wide range of cellular activities. Researchers in cytoskeleton studies such as cell mechanics and motility, and the emerging “dynamic structural biology” field will significantly benefit from these findings.
Perspectives and future challenges
The central challenge to studying “protein dynamics” in real-time lies in bridging the gap in time scales: HS-AFM captures dynamics of two-dimensional surface structure of proteins within the milliseconds to seconds range, whereas molecular dynamics (MD) simulations typically explore atomistic structure and operate within the femtoseconds to microseconds domain. Protein dynamics encompass a spectrum of temporal scales, from atomic vibrations to molecular tumbling and collective motions in simulations. HS-AFM stands out as a potent technique for delving into protein dynamics, including processes like protein folding and conformational changes triggered by drugs or protein interactions. Additionally, a significant limitation of MD simulation is the spatial modeling constraint, which restricts the study of large, complex biological systems. However, utilizing HS-AFM enables the construction of intricate protein models, facilitating the high-speed imaging of their structures and dynamics during functional activity. Thus, addressing the temporal and spatial resolution discrepancies between experimental data and simulation data necessitates a comprehensive strategy that allows for concurrent observation of protein structures, dynamics, and functions at the ultrafast/real-time and atomistic levels, surpassing individual methods.
Funder
The authors express their gratitude for the facility and financial support provided by the World Premier International Research Center Initiative (WPI), MEXT, Japan, as well as the instrumental assistance provided by Toshio Ando, WPI-NanoLSI, Kanazawa University. The authors also acknowledge the financial support from KAKENHI (Japan Society for the Promotion of Science) for K.X.N. (19K06581, 23K05713, 23H02452-01) and T.Q.P.U. (#23H02452) and N.K. (20H00327); and CREST, Japan Science and Technology Agency (JPMJCR1762 to N.K.).
About Nano Life Science Institute (WPI-NanoLSI), Kanazawa University
Understanding nanoscale mechanisms of life phenomena by exploring “uncharted nano-realms”
Cells are the basic units of almost all life forms. We are developing nanoprobe technologies that allow direct imaging, analysis, and manipulation of the behavior and dynamics of important macromolecules in living organisms, such as proteins and nucleic acids, at the surface and interior of cells. We aim at acquiring a fundamental understanding of the various life phenomena at the nanoscale.
About the World Premier International Research Center Initiative (WPI)
The WPI program was launched in 2007 by Japan’s Ministry of Education, Culture, Sports, Science and Technology (MEXT) to foster globally visible research centers boasting the highest standards and outstanding research environments. Numbering more than a dozen and operating at institutions throughout the country, these centers are given a high degree of autonomy, allowing them to engage in innovative modes of management and research. The program is administered by the Japan Society for the Promotion of Science (JSPS).
See the latest research news from the centers at the WPI News Portal:
Main WPI program site:
www.jsps.go.jp/english/e-toplevel
About Kanazawa University
As the leading comprehensive university on the Sea of Japan coast, Kanazawa University has contributed greatly to higher education and academic research in Japan since it was founded in 1949. The University has three colleges and 17 schools offering courses in subjects that include medicine, computer engineering, and humanities.
The University is located on the coast of the Sea of Japan in Kanazawa – a city rich in history and culture. The city of Kanazawa has a highly respected intellectual profile since the time of the fiefdom (1598-1867). Kanazawa University is divided into two main campuses: Kakuma and Takaramachi for its approximately 10,200 students including 600 from overseas.
Journal
eLife
Article Title
Deciphering the actin structure-dependent preferential cooperative binding of cofilin
Article Publication Date
2-Aug-2024