FOR IMMEDIATE RELEASE
Credit: Twomey lab, Johns Hopkins Medicine
FOR IMMEDIATE RELEASE
By super cooling a molecule on the surface of brain cells down to about minus 180 degrees Celsius — nearly twice as cold as the coldest places in Antarctica — scientists at Johns Hopkins Medicine say they have determined how a widely-used epilepsy drug works to dampen the excitability of brain cells and help to control, although not cure, seizures.
The research, published June 4 in Nature Structural & Molecular Biology, identifies critical connections between activity of the epilepsy drug perampanel and the resulting movements of the AMPA receptor — a brain cell surface molecule. The researchers say the findings could eventually help with designing new drugs that target the receptor to treat other neurological conditions such as Alzheimer’s disease, schizophrenia, learning disabilities, brain cancers called glioblastoma and chronic pain.
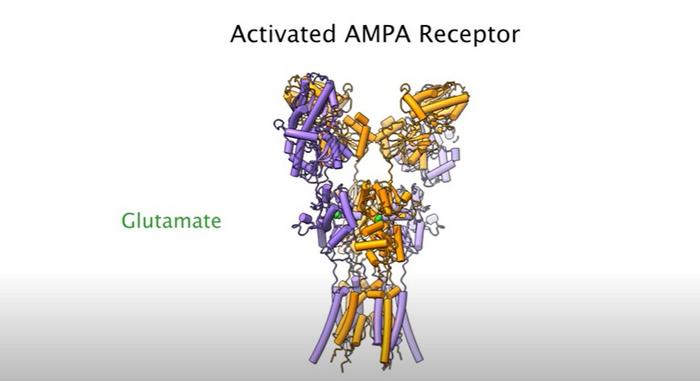
The AMPA receptor plays a critical role for one of the brain’s most abundant neurotransmitters — glutamate — which activates brain cells (neurons) by connecting with a protein on the cell surface (AMPA) in a Pac-man-like connection, in which AMPA receptors engulf glutamate.
Up to four glutamate molecules can bind to a single AMPA receptor. The connection enables a flood of ions (positively-charged particles) to enter the neuron and activate (excite) it.
“AMPA receptors and glutamate are necessary for most aspects of life, including the processes of learning, memory and encoding experiences,” says Edward Twomey, Ph.D., assistant professor of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine. “Most neurological diseases trace back in some way to AMPA receptors and glutamate.”
Twomey was approached by neuroscientist Richard Huganir, Ph.D., who has been studying AMPA receptors for 40 years, to collaborate on research to better understand the receptors’ structure and their glutamate binding process.
Overactivation (excitation) of AMPA receptors is known to cause epilepsy. Perampanel, which targets the AMPA receptor, is the only medicine approved by the U.S. Food and Drug Administration to target AMPA receptors, but many pharmaceutical companies are working on similar compounds, the researchers say.
“This drug was initially discovered in the 1980s, and its precise mechanism has been a long standing mystery,” says Twomey.
“We know that this drug doesn’t block or get stuck in the receptor’s ion channels,” says Huganir, Bloomberg Distinguished Professor of Neuroscience and Psychological and Brain Sciences and director of the Solomon H. Snyder Department of Neuroscience. Other scientists had found where perampanel binds to AMPA receptors in pockets around the ion channel but had not found the way that connection disrupts ion flow.
To study the mechanism, the researchers turned to cryo-electron microscopy (cryoEM), which has evolved in the last two decades as a powerful tool to study structures a million times smaller than the width of a human hair.
Johns Hopkins postdoctoral fellow W. Dylan Hale, Ph.D., working in the Twomey and Huganir labs, performed most of the experiments and analysis in the Beckman Center for CryoEM at Johns Hopkins, where they super chill biological molecules and take images at various timepoints.
For the study, the researchers analyzed millions of images of AMPA receptors in brain cells from mice and rat models and their interaction with the originally discovered version of the perampanel drug, GYKI-52466. “These interactions happen at a super tiny scale, about 1 to 2 angstroms,” says Twomey. An angstrom is 1 10 billionth of a meter.
They looked at the GYKI-52466 drug’s binding, with and without glutamate. They also performed electrical recordings of the ion flow, and physiology studies in mice to complement the cryoEM images.
The scientists used artificial intelligence and machine learning tools to average and combine the cryoEM images into a 3D reconstruction of the receptor.
When glutamate binds to the AMPA receptor in one of four positions, a strand of the receptor comes down and pulls open the ion channel enabling the flow of ions, akin to how a pull chain releases water from a shower head.
The researchers found that two of the four glutamate binding positions are the most important in the GYKI-52466 drug’s ability to block the ion flow.
“The drug binds to the AMPA receptor and prevents the ion channel from opening by blocking the ability of glutamate to pull on the strand that opens the ion channel,” says Twomey. “It seems to decouple the glutamate binding regions from each other and put the receptor into a desensitized state.”
Huganir also plans to work with Twomey to use cryoEM to study what happens to the AMPA receptor when it’s mutated.
“We want to know what’s wrong with the receptor’s structure that disables its function,” Huganir says. “In theory, we could develop drugs to make the receptor more active to treat conditions in which the receptor’s structure is altered.”
In addition to Hale, Twomey and Huganir, researchers who contributed to the work are Alejandra Montaño Romero and Albert Lau at Johns Hopkins, and Cuauhtemoc Gonzalez and Vasanthi Jayaraman at the University of Texas Health Science Center at Houston.
Funding support for the study was provided by the Searle Scholars Program, the Diana Helis Henry Medical Research Foundation, and the National Institutes of Health (R37 NS036715, R01 GM094495, R35 GM122528, F99NS130928, K99 MH132811).
DOI: 10.1038/s41594-024-01328-0
Journal
Nature Structural & Molecular Biology
Discover more from Science
Subscribe to get the latest posts sent to your email.


