Recent studies highlighted in the scientific community are underscoring the importance of specific molecular markers in predicting the prognosis of various cancers. Among these, a notable study published in the Journal of Translational Medicine investigates the role of SLC16A3 as an immunosuppressive marker in Kupffer cells concerning hepatocellular carcinoma (HCC) in patients with Hepatitis B Virus (HBV) infections. The findings have significant implications for both clinical practice and future research directions.
Hepatocellular carcinoma remains one of the most prevalent forms of cancer globally, particularly in regions with high endemic rates of HBV. The complexity of HCC is exacerbated by its association with chronic liver diseases, including cirrhosis and liver fibrosis, prompting a need for effective prognostic biomarkers. Traditional histopathological assessments, while important, often do not capture the full spectrum of biological behavior exhibited by HCC. Hence, the identification and validation of new prognostic markers have become paramount in enhancing patient outcomes and tailoring therapeutic strategies.
Marking a significant advancement in this field, the study led by Zhang et al. focuses on SLC16A3, which encodes a member of the monocarboxylic acid transporter family primarily expressed in various tissues, including the liver. The hypothesis of the research posits that high expression levels of SLC16A3 in Kupffer cells, which are liver-resident macrophages responsible for immune surveillance, may correlate with an immunosuppressive tumor microenvironment. This association could hinder the body’s antitumor immune responses, ultimately leading to poorer prognoses for affected patients.
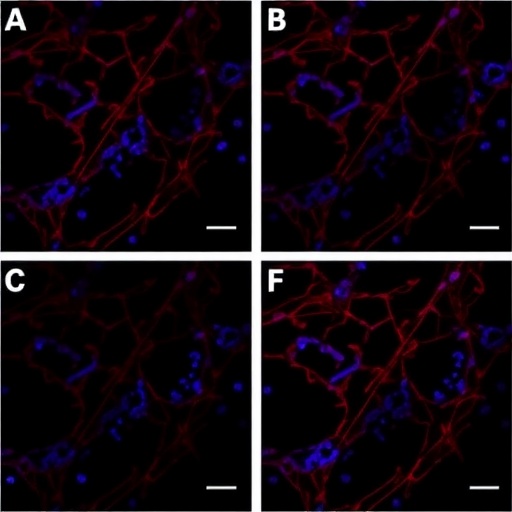
The authors meticulously analyzed tissue samples from patients diagnosed with HBV-positive HCC to assess SLC16A3 expression levels. The study utilized advanced immunohistochemical techniques, allowing for precise localization and quantification of the protein within liver tissues. Their results revealed a pronounced expression of SLC16A3 in Kupffer cells adjacent to tumor regions as opposed to non-cancerous liver tissues. This differential expression pattern raises important questions regarding the role of these immune cells in facilitating tumor progression and immune evasion.
Moreover, the study investigated the relationship between SLC16A3 expression levels and various clinical parameters including tumor staging, lymph node involvement, and patient survival rates. They found that elevated SLC16A3 expression correlated with advanced tumor stages and a significant reduction in overall survival, suggesting its utility as a dynamic prognostic biomarker. This represents a pivotal finding, revealing a direct link between an immune cell marker and clinical outcomes in patients afflicted with HCC.
Following the meticulous evaluation of SLC16A3 as a prognostic marker, the researchers further explored its underlying mechanisms. They proposed that the immunosuppressive environment fostered by Kupffer cells expressing high levels of SLC16A3 could impair the activation and proliferation of T cells, crucial components of the body’s adaptive immune response. By inhibiting T cell functions, these immunosuppressive macrophages may permit the tumor to grow and metastasize without being effectively targeted by the immune system.
In their conclusion, Zhang and colleagues emphasized the necessity of understanding the intricacies of the tumor microenvironment in the context of HBV-positive HCC. They advocate for further studies to delineate the mechanisms by which SLC16A3 contributes to immunosuppression and its potential as a therapeutic target. The study signifies an exciting intersection of immunology and oncology, with the promise of delivering novel strategies to enhance anti-tumor immunity and improve patient outcomes in the sphere of liver malignancies.
The implications of SLC16A3 extend beyond prognostication, as the researchers hint at future therapeutic innovations that might arise from manipulating its pathways. Potential strategies could aim at reversing the immunosuppressive effects in the tumor microenvironment by targeting SLC16A3 specifically. This may open new frontiers for immunotherapy approaches, in conjunction with established treatments, to bolster the immune system’s capacity to eradicate HCC.
In summary, the research conducted by Zhang et al. contributes significantly to our understanding of biomarker applications in HBV-positive liver cancer. Their findings advocate for the need to pivot towards immunological perspectives in treating HCC, emphasizing that the immune landscape within tumors can dictate patient prognosis and treatment success.
By elucidating the role of SLC16A3 in Kupffer cells, this study lays the groundwork for further investigations aimed at developing innovative cancer therapies that harness the immune system. The translation of these findings into clinical practice has the potential to redefine management strategies for patients with hepatocellular carcinoma, particularly among those with a chronic viral background.
Exploring the broader context, the emphasis on molecular markers like SLC16A3 underlines a paradigm shift in cancer diagnostics and treatment. As we continue to unravel the complexities of HCC and its immunological interactions, the pathway for enhancing survival rates becomes clearer, illustrating the significance of continuous research in this critical area.
Collaborating efforts across multiple disciplines, including oncology, immunology, and molecular biology, are essential to transform these early findings into clinical realities. As our understanding deepens, we must remain vigilant in ensuring that innovations are rapidly translated to patient care, underscoring the hope for a future where HBV-positive HCC is met with improved prognostic strategies and treatment outcomes.
Strong interdisciplinary collaborations, innovative research designs, and robust clinical trials will be pivotal in validating the prognostic value of SLC16A3 and exploring its role as a therapeutic target. As researchers embark on this journey, the collective aim remains clear—to improve the quality of life and survival chances for patients grappling with the burden of hepatocellular carcinoma.
The exploration of SLC16A3 in the context of HBV-positive hepatocellular carcinoma is not just a testament to scientific endeavor but also reflects the relentless pursuit of knowledge that drives medical sciences forward. Each finding adds a piece to the intricate puzzle of cancer biology, and with each study, we inch closer to groundbreaking interventions that can profoundly change the landscape of cancer therapeutics.
Subject of Research: The role of SLC16A3 as an immunosuppressive marker in Kupffer cells and its prognostic significance in HBV-positive hepatocellular carcinoma.
Article Title: SLC16A3 as an immunosuppressive Kupffer cell marker predicts poor prognosis in HBV-positive hepatocellular carcinoma.
Article References:
Zhang, J., Pan, Y., Zhao, Z. et al. SLC16A3 as an immunosuppressive Kupffer cell marker predicts poor prognosis in HBV-positive hepatocellular carcinoma.
J Transl Med 23, 988 (2025). https://doi.org/10.1186/s12967-025-06861-0
Image Credits: AI Generated
DOI:
Keywords: immunosuppressive marker, SLC16A3, Kupffer cells, hepatocellular carcinoma, HBV, prognostic biomarker, immune evasion, cancer therapy.