In a groundbreaking study published in Nature, Chinese paleontologists have unveiled unprecedented insights into the complex evolutionary journey that reshaped the jaw and ear structures of early mammals. By employing cutting-edge high-resolution computed tomography (CT) scans on two historically significant fossil specimens, the research team led by Professor Fangyuan Mao from the Institute of Vertebrate Paleontology and Paleoanthropology at the Chinese Academy of Sciences has illuminated a detailed four-stage morphological sequence. This sequence not only refines our understanding of the mammalian cranio-mandibular joint evolution but also elucidates the gradual functional specialization separating chewing mechanics from auditory capabilities.
The mammalian cranio-mandibular joint, characterized by the dentary condyle articulating with the squamosal glenoid fossa, represents a pivotal vertebrate innovation. This secondary jaw joint supplanted the ancestral reptilian articular-quadrate joint, fundamentally altering the biomechanics of feeding and hearing. Despite its importance, the transitional morphological states bridging advanced cynodonts and early mammals have remained elusive, hampered by a sparse fossil record and the subtlety of intermediary anatomical structures. The employment of non-destructive CT scanning technology has revolutionized the reevaluation of these fossils, revealing hitherto hidden joint configurations and clarifying the evolutionary narrative.
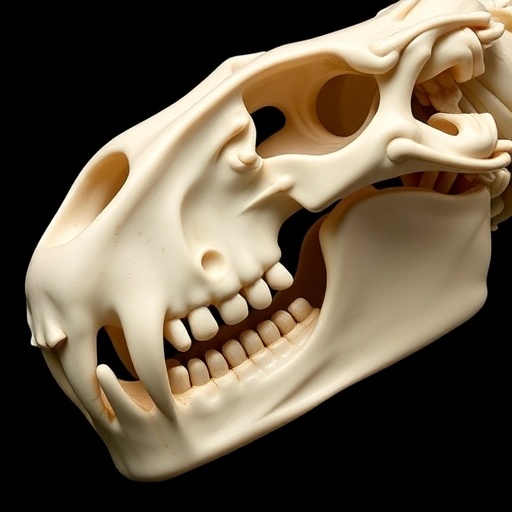
One of the two fossil specimens reevaluated is Polistodon chuannanensis, a Middle Jurassic tritylodontid originally described in 1984 from the Zigong region in Sichuan. The CT data overturned previous assumptions by exposing a unique dentary condyle–jugal fossa secondary joint, a configuration unprecedented among tetrapods. This discovery challenges long-held paradigms about secondary jaw joint morphology and extends conceptual frameworks regarding functional adaptations associated with diverse ecological niches, particularly the interaction between cranial biomechanics and lifestyle.
The second specimen, uncovered from the Lower Jurassic strata in Lufeng, Yunnan, represents a newly identified morganucodontan genus and species named Camurocondylus lufengensis. The simplicity of its dentary condyle, formed by an upward bending of the posterior portion of the dentary lateral ridge, offers compelling evidence supporting hypotheses that mammalian dentary condyles evolved directly from the lateral ridge structure. This anatomical configuration underscores a gradual modification of jaw load-bearing surfaces that paved the way for further diversification within early mammaliaforms.
Synthesizing these morphologies allowed the researchers to propose a refined four-stage evolutionary model. The initial stage retains the ancestral reptilian articular–quadrate joint. Stage two involves advanced cynodonts manifesting a dominant primitive joint complemented by emerging secondary contact points between other cranial elements. Stage three is marked by stem mammaliaforms where the secondary joint assumes the primary load-bearing role, while the primitive joint transitions to facilitate sound transmission. Stage four culminates in mammals and their close relatives—docodonts and haramiyidans—where a fully developed dentary–squamosal joint exists concomitantly with the primitive joint’s transformation into the middle ear ossicular chain.
Intriguingly, the findings suggest that diverse jaw joint types arose independently multiple times during evolutionary history, rather than following a single linear trajectory. While the dentary–squamosal articulation is not strictly unique to mammals, the evolution of its load-bearing variant appears as a hallmark of mammaliaforms. The dentary–zygomatic (or jugal) joint observed in Polistodon exemplifies a specialized adaptation likely linked to its herbivorous diet and fossorial (burrowing) behavior. This adaptation illustrates how ecological pressures can drive morphological innovation distinct from generalized evolutionary pathways.
Comparative analysis of Camurocondylus and Polistodon further details disparate evolutionary drivers behind these jaw specializations. The “miniaturization drive hypothesis,” proposing that small body size and insectivory catalyze miniaturization and morphological shifts conducive to auditory refinement, aptly explains the evolution of Camurocondylus. Conversely, Polistodon, larger-bodied and herbivorous, suggests alternative forces at play, including adaptation to subterranean lifestyles. These behavioral and ecological distinctions correlate with paleontological evidence hinting at tritylodontid burrow systems near the site of the Polistodon specimen, emphasizing environmental influences on phenotypic expression.
The authors propose phenotypic plasticity, defined as environmentally induced morphological variation, as a significant evolutionary mechanism promoting secondary joint diversification in late cynodonts. This paradigm advocates that external ecological factors, alongside genetic and developmental processes, synergistically shaped the skull and jaw architectures essential for the specialized functions observed in mammals. The incorporation of plasticity into evolutionary models marks a progressive step towards appreciating the interplay between organismal development and ecological context.
This research not only enhances comprehension of the origins and diversification of the mammalian jaw joint but also provides a comprehensive framework for interpreting the evolutionary partitioning of feeding and auditory functions. By tracing the anatomical transitions from load-bearing primitive joints to sophisticated auditory ossicles, the study amplifies our grasp of the intricate co-evolutionary dynamics within vertebrate cranial systems.
Beyond anatomical novelty, the evolutionary narrative encapsulated in these fossils spotlights broader biological principles. Variations in joint morphology reflect functional trade-offs, ecological adaptations, and developmental constraints that collectively sculpt the vertebrate lineage. The nuanced interplay between these factors culminated in the advent of the mammalian middle ear and the modern chewing apparatus, hallmark features underpinning mammalian success.
By merging paleontological evidence with advanced imaging techniques, Professor Mao and colleagues set a benchmark for future explorations of vertebrate history. Their multidisciplinary approach leverages fossil reinterpretation to reconstruct nuances of form and function invisible under traditional methodologies. Importantly, these findings invigorate discussions about the timing, mechanisms, and ecological drivers shaping pivotal vertebrate innovations.
In sum, this study profoundly enriches our evolutionary narrative, positioning the mammalian jaw joint as a dynamic product of incremental modifications, ecological pressures, and developmental plasticity. Such revelations deepen scientific appreciation of vertebrate morphological evolution, offering a nuanced perspective on how complex biological systems emerge through time.
Subject of Research: Evolution of mammalian jaw and ear joints in vertebrates
Article Title: Not explicitly provided in content
News Publication Date: September 24, [Year not specified but presumably 2025 based on DOI]
Web References:
https://doi.org/10.1038/s41586-025-09572-0
References: Published in Nature on September 24, lead author Prof. Fangyuan Mao et al.
Image Credits: Not provided
Keywords: Evolutionary developmental biology, Evolutionary processes, Fossils