· Lupus is an autoimmune disease affecting more than 1.5 million people in the U.S.
· Lupus is an autoimmune disease affecting more than 1.5 million people in the U.S.
· Until now, the causes of this disease remained unclear
· Scientists are working to expand research into novel treatment
CHICAGO — Northwestern Medicine and Brigham and Women’s Hospital scientists have discovered a molecular defect that promotes the pathologic immune response in systemic lupus erythematosus (known as lupus) and show that reversing this defect may potentially reverse the disease.
Lupus affects more than 1.5 million people in the U.S. Until this new study, the causes of this disease were unclear. Lupus can result in life-threatening damage to multiple organs including the kidneys, brain and heart. Existing treatments often fail to control the disease, the study authors said, and have unintended side effects of reducing the immune system’s ability to fight infections.
“Up until this point, all therapy for lupus is a blunt instrument. It’s broad immunosuppression,” said co-corresponding author Dr. Jaehyuk Choi, associate professor of dermatology at Northwestern University Feinberg School of Medicine and a Northwestern Medicine dermatologist. “By identifying a cause for this disease, we have found a potential cure that will not have the side effects of current therapies.”
“We’ve identified a fundamental imbalance in the immune responses that patients with lupus make, and we’ve defined specific mediators that can correct this imbalance to dampen the pathologic autoimmune response,” said co-corresponding author Dr. Deepak Rao, an assistant professor of medicine at Harvard Medical School and a rheumatologist at Brigham and Women’s Hospital and co-director of its Center for Cellular Profiling.
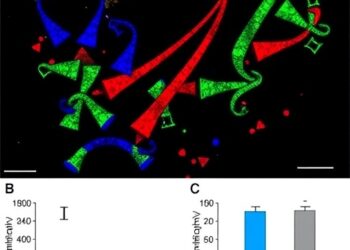
In a study to be published in Nature on July 10, the scientists report a new pathway that drives disease in lupus. There are disease-associated changes in multiple molecules in the blood of patients with lupus. Ultimately, these changes lead to insufficient activation of a pathway controlled by the aryl hydrocarbon receptor (AHR), which regulates cells’ response to environmental pollutants, bacteria or metabolites. Insufficient activation of AHR results in too many disease-promoting immune cells, called the T peripheral helper cells, that promote the production of disease-causing autoantibodies.
To show this discovery can be leveraged for treatments, the investigators returned the aryl hydrocarbon receptor-activating molecules to blood samples from lupus patients. This seemed to reprogram these lupus-causing cells into a cell called a Th22 cell that may promote wound healing from the damage caused by this autoimmune disease.
“We found that if we either activate the AHR pathway with small molecule activators or limit the pathologically excessive interferon in the blood, we can reduce the number of these disease-causing cells,” said Choi, also the Jack W. Graffin Professor at Feinberg. “If these effects are durable, this may be a potential cure.”
Choi, Rao and colleagues next want to expand their efforts into developing novel treatments for lupus patients. They are now working to find ways to deliver these molecules safely and effectively to people.
Other Northwestern authors are first author Calvin Law, Arundhati Pillai, Brandon Hancock and Dr. Judd Hultquist. Brigham and Women’s Hospital authors include Vanessa Sue Wacleche, Ye Cao, John Sowerby, Alice Horisberger, Sabrina Bracero, Ifeoluwakiisi Adejoorin, Eilish Dillon, Daimon Simmons, Elena Massarotti, Karen Costenbader, Michael Brenner and James Lederer.
The title of the article is “Interferon subverts an AHR-JUN axis to promote CXCL13+ T cells in lupus.”
The research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants K08 AR072791, P30 AR070253, R01 AR078769 and P30 AR075049; National Institute of Allergy and Infectious Diseases grants R01 AI176599, P30 AI117943, R01 AI165236 and U54 AI170792; National Cancer Institute grants F31 CA268839 and CA060553, all of the National Institutes of Health (NIH); and NIH Director’s New Innovator Grant 1DP2AI136599-01, and grants from Lupus Research Alliance, Burroughs Wellcome Fund, Bakewell Foundation, Leukemia and Lymphoma Society and American Cancer Society.
Journal
Nature
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
“Interferon subverts an AHR-JUN axis to promote CXCL13+ T cells in lupus.”
Article Publication Date
10-Jul-2024