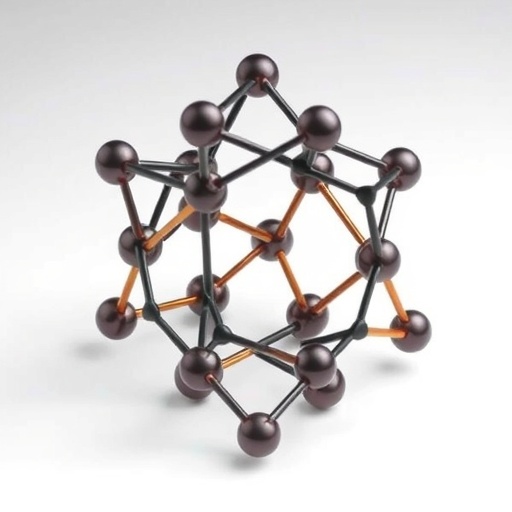
In an extraordinary breakthrough that stands to reshape the landscape of carbon chemistry, a team of chemists from Oxford University has revealed the synthesis and stabilization of a novel molecular form of carbon known as cyclo[48]carbon. This molecule, composed of 48 carbon atoms arranged in a unique alternating single and triple bond pattern, has been stabilized in liquid solution at room temperature—a feat long regarded as a monumental challenge within the field. Unlike prior examples of cyclocarbons, which could typically only be studied under either gaseous phases or extreme cryogenic temperatures, cyclo[48]carbon defies these constraints, marking a new frontier for the exploration of carbon allotropes under normal laboratory conditions.
The newly synthesized cyclo[48]carbon exists not as a lonely ring but rather as a [4]catenane structure; in other words, the cyclic carbon framework is mechanically interlocked with three other macrocyclic molecules serving as protective rings. This catenane architecture plays a critical role by effectively shielding the fragile carbon ring from environmental degradation, thereby enhancing its stability significantly. This approach marks a notable deviation in synthetic strategy compared to previous attempts, highlighting the importance of molecular design in manipulating both the reactivity and resilience of elusive molecular carbon allotropes.
Traditional cyclocarbon rings have long fascinated chemists due to their predicted electronic properties and unusual bonding patterns; however, their inherent instability has restricted their study to either the gas phase or ultra-low temperatures near absolute zero. The present achievement stands out because the cyclo[48]carbon catenane remains intact and characterizable at ambient conditions, with a remarkable half-life of 92 hours in solution at 20°C. Such stability opens the door for extensive studies into the chemical behavior, reactivity, and potential applications of cyclocarbon molecules, potentially spurring advances in materials science, nanoengineering, and molecular electronics.
The synthesis pathway developed by the researchers involved a meticulous design aimed at minimizing ring strain while implementing mild reaction conditions that avoid the decomposition of sensitive intermediates. By selecting a sufficiently large cyclocarbon ring—one with reduced inherent strain—the team could navigate one of the primary obstacles to stability that smaller cyclocarbons face. The unmasking step, where a precursor molecule is chemically transformed into the cyclo[48]carbon catenane, was particularly delicate, requiring finely tuned reaction parameters to preserve the structural integrity of the molecule.
Characterization of this unprecedented molecular entity leveraged a suite of advanced spectroscopic techniques, offering compelling evidence for its structure. Mass spectrometry confirmed the molecular weight consistent with the C_48 ring, while ultraviolet-visible spectroscopy provided insights into its electronic transitions. Raman spectroscopy offered additional vibrational data reinforcing the bonding framework. Perhaps most strikingly, nuclear magnetic resonance (NMR) spectroscopy revealed a singular intense resonance in the carbon-13 spectrum, indicating that all 48 sp^1 carbons experience equivalent chemical environments. This uniformity is consistent with a symmetric, well-defined cyclocarbon catenane architecture, validating the success of the synthetic strategy.
Dr. Yueze Gao, the lead author and a rising figure in the Oxford Department of Chemistry, emphasized the transformative nature of this discovery. He underscored that the ability to stabilize cyclocarbons in ambient solution conditions is a fundamental step that will ease experimental investigations into their properties and reactivity. Such accessibility paves the way for pioneering research that could elucidate new chemical phenomena and inform the design of novel molecular devices based on these exotic carbon frameworks.
Professor Harry Anderson, the senior author overseeing this project, reflected on the long journey toward this milestone. Citing initial proposals and preliminary work dating from 2012 to 2015, he remarked on the perseverance required to realize these achievements. Recognizing the exceptional NMR facilities at Oxford, Anderson acknowledged the environment’s crucial role in enabling this research. The synthesis of cyclocarbon catenanes stable at room temperature had once seemed quixotic, but with rigorous experimentation and innovation, the dream has been realized.
Collaborations extended beyond Oxford, involving the University of Manchester, the University of Bristol, and the Central Laser Facility at Rutherford Appleton Laboratory. The interdisciplinary nature of the team and their utilization of world-class instruments underscore the complexity and sophistication demanded by this research. The combination of synthetic chemistry, physical characterization, and state-of-the-art instrumentation reflects the high bar set for studying novel carbon allotropes.
Placing this accomplishment in context reveals its profound significance. Until now, the only new molecular carbon allotrope to be practically studied under ambient conditions was the fullerene, discovered in the early 1990s, which revolutionized nanomaterials and carbon science. Cyclo[48]carbon offers a distinctly different structural motif, featuring a conjugated ring of carbon atoms with alternating single and triple bonds, whose electronic properties are predicted to differ fundamentally from those of fullerenes and graphene. The stable, solution-phase study of such molecules may unlock unforeseen chemical reactivity and functionalities.
The ramifications for future research are substantial. With cyclo[48]carbon and its catenane protection strategy now proven viable, the stage is set for the synthesis of other cyclocarbons with varied sizes and topologies. Researchers may explore their potential as molecular wires, quantum materials, or building blocks for supramolecular assemblies. The concept of mechanical interlocking to control molecular stability introduces a valuable design paradigm likely to inspire analogous approaches in other sensitive molecular systems.
The publication detailing this work, titled “Solution-phase stabilization of a cyclocarbon by catenane formation,” has been featured in Science, offering the scientific community a detailed account of the synthetic methodologies, spectroscopic data, and theoretical underpinnings that make this discovery possible. This contribution enriches our understanding of carbon chemistry’s frontiers and heralds a new chapter for molecular design emerging from the interplay between synthetic ingenuity and characterization prowess.
As laboratories worldwide digest this landmark finding, the chemical community anticipates a surge in innovative research into cyclocarbons and related nanostructures. The delicate balance of bond formation, strain relief, and mechanical stabilization exemplified by this study may well become a cornerstone for manipulating unstable molecular species, helping to bridge the gap between theoretical predictions and practical chemical realities. In this way, the stabilized cyclo[48]carbon catenane could catalyze future discoveries spanning materials science, nanotechnology, and quantum chemistry.
In summary, the synthesis and ambient stabilization of cyclo[48]carbon represent a breakthrough poised to expand the molecular toolbox of carbon allotropes accessible to chemists. It demonstrates that by marrying clever synthetic tactics with advanced spectroscopic exploration, elusive molecules previously confined to conceptual boundaries can now be probed and harnessed. This achievement heralds a promising era wherein the chemistry of carbon, the backbone of life and technology, reveals ever more layers of complexity and potential.
Subject of Research: Synthesis and solution-phase stabilization of cyclo[48]carbon via catenane formation
Article Title: Solution-phase stabilization of a cyclocarbon by catenane formation
News Publication Date: 14 August 2025
Web References:
- DOI: 10.1126/science.ady6054
- Oxford Department of Chemistry: https://www.chem.ox.ac.uk/
- Central Laser Facility: https://www.clf.stfc.ac.uk/Pages/home.aspx
References:
- Krätschmer et al., Fullerene synthesis (Nature, 1990): https://doi.org/10.1038/347354a0
Image Credits: Harry Anderson
Keywords
Cyclo[48]carbon, Carbon allotrope, Cyclocarbon catenane, Molecular synthesis, Room temperature stability, Nuclear magnetic resonance, Mass spectrometry, Raman spectroscopy, Macrocyclic threading, Chemical bonding, Molecular electronics, Advanced materials