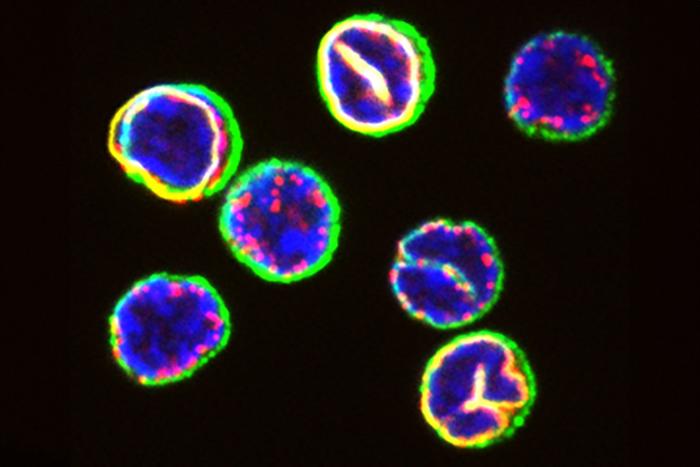
From the outside, most T cells look the same: small and spherical. Now, a team of researchers led by Berend Snijder from the Institute of Molecular Systems Biology at ETH Zurich has taken a closer look inside these cells using advanced techniques. Their findings show that the subcellular spatial organisation of cytotoxic T cells – which Snijder refers to as their cellular architecture – has a major influence on their fate.

Credit: Ben Hale / ETH Zurich
From the outside, most T cells look the same: small and spherical. Now, a team of researchers led by Berend Snijder from the Institute of Molecular Systems Biology at ETH Zurich has taken a closer look inside these cells using advanced techniques. Their findings show that the subcellular spatial organisation of cytotoxic T cells – which Snijder refers to as their cellular architecture – has a major influence on their fate.
Characteristics that determine a cell’s fate
When cells with nuclear invaginations encounter a pathogen, they turn into powerful effector cells that rapidly proliferate and kill the pathogen. Their fellow cells with a spherical nucleus – that is, with no nuclear invaginations – evolve at a more leisurely pace: they take longer to activate and eventually differentiate into long-lived memory cells that defend the organism against future attacks by the same pathogen.
Scientists identified these two functionally distinct populations of T cells some 50 years ago. “But until now we weren’t sure which characteristics determined whether a T cell would become an effector cell or a memory cell,” says Ben Hale, a postdoc in Snijder’s research group and lead author of the article that recently appeared in the journal Science.
To help identify these characteristics, the researchers developed a platform that automatically analyses microscopy images of immune cells. They then presented this platform with thousands of T cells from 24 healthy volunteers who donated their blood to the Zurich Blood Donation Service of the Swiss Red Cross.
Unexpected differences
Using a machine-learning approach, the platform classified the cells into three different groups. “We’d already seen how some T cells appear bottle-shaped when activated,” Snijder says. “But we didn’t expect the platform to split the round cells into two different groups.”
On further investigation, the researchers also discovered that the differences in cellular architecture between the two classes of round cells also has a functional significance. “The cells with nuclear invaginations are designed to activate rapidly: many of them convert into bottle-shaped effector cells within 24 hours,” Hale says.
“They also mount a stronger response when activated – and they proliferate much faster than cells without nuclear invaginations,” Snijder adds. He and his team also identified the molecular mechanism that leads to the faster and stronger activation of cells with nuclear invaginations: “Their special cellular architecture enables a heightened influx of calcium ions,” Snijder says.
Both researchers emphasise that there are still many questions to be answered. For example, Snijder and his team now hope to discover how the organism consistently ensures that around 60 percent of cytotoxic T cells in the blood have nuclear invaginations, while 35 percent have no invaginations and the remaining 5 percent are bottle-shaped.
Making therapies more clinically effective
Snijder and Hale note that their results are not only “important for gaining a better understanding of how our immune cells work”, but also play a crucial role in the fight against cancer, for example: “Many novel therapies use T cells to kill cancer cells,” Snijder says. “If we can find a way to specifically select and deploy these cellular architectures, we may be able to improve the clinical efficacy of such therapies.”
Journal
Science
Article Title
Cellular architecture shapes the naïve T cell response
Article Publication Date
6-Jun-2024