In the relentless quest to uncover the neural underpinnings of addiction, a groundbreaking new study has illuminated a critical brain circuit alteration in individuals dependent on opioids. Researchers have identified a markedly blunted response within the anterior midcingulate cortex (aMCC), a region pivotal to reward processing, decision-making, and adaptive behavior. What elevates this discovery beyond observation is the demonstration that this impaired neural activity can be normalized through targeted non-invasive brain stimulation, specifically prefrontal transcranial magnetic stimulation (TMS). The research, recently published in Translational Psychiatry, offers a promising avenue for novel therapeutic interventions aimed at the deep-seated neurobiological disruptions characteristic of opioid use disorder (OUD).
Opioid addiction remains a devastating public health crisis worldwide, with profound psychological, social, and economic repercussions. Despite the availability of pharmacological treatments such as methadone and buprenorphine, relapse rates remain high and effective long-term recovery solutions are elusive. Central to the pathology of addiction is the dysfunction of reward processing circuits, which impairs an individual’s ability to experience pleasure and makes drug-seeking behavior compulsive rather than voluntary. While much of prior research has focused on the dopamine system and limbic structures, this study shifts focus to the aMCC, highlighting its crucial role in mediating responses to reward stimuli in opioid users.
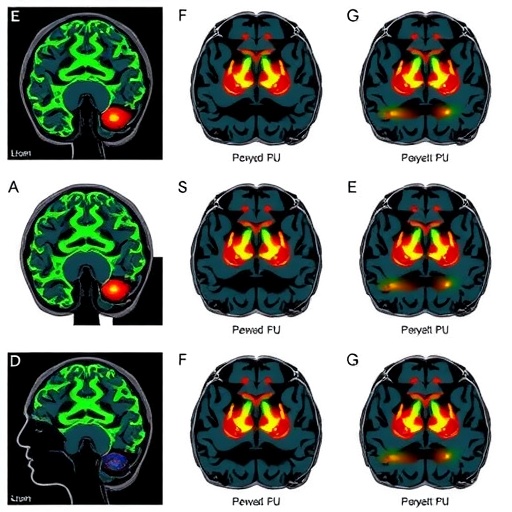
The anterior midcingulate cortex straddles the intersection of cognitive control, pain perception, and motivational aspects of behavior. Functionally, it acts as an integrative hub, processing signals related to reward expectation and error monitoring. In opioid-dependent individuals, this study documents that the aMCC response to rewarding stimuli is significantly attenuated—a phenomenon that explains, in part, the diminished sensitivity to natural reinforcers observed clinically. Utilizing functional magnetic resonance imaging (fMRI) during reward-based tasks, researchers recorded neural activations, revealing that, compared to healthy controls, opioid users consistently exhibited lower aMCC activation in response to monetary incentives.
To interrogate whether this neural deficit is reversible, the researchers applied repetitive TMS over the dorsolateral prefrontal cortex (DLPFC), a brain region interconnected with the aMCC and implicated in executive control and regulation over limbic circuits. TMS utilizes magnetic pulses to induce electric fields in targeted cortical areas, transiently enhancing or suppressing neuronal excitability. In this cohort of opioid users, daily sessions of prefrontal TMS were delivered over a two-week period. Post-treatment neuroimaging revealed a remarkable restoration of aMCC activity during reward tasks, bringing the neural signature closer to that observed in non-addicted individuals.
What cements the significance of this study is the demonstration that the normalization of brain activity was coupled with improved behavioral indices related to reward sensitivity and reduced drug craving. Participants reported decreased urges to use opioids and showed improved performance in decision-making paradigms, which rely heavily on intact reward circuits. These findings suggest that TMS-induced modulation of frontocingulate networks can recalibrate reward processing deficits, offering a potential adjunct or alternative to pharmacotherapy in OUD.
The mechanistic insights derived from this work extend beyond mere correlation, proposing a causal pathway wherein disrupted communication between the prefrontal cortex and aMCC underlies impaired reward responsiveness in opioid addiction. By dampening the cortical hypoactivity through external magnetic stimulation, it is possible to reinstate normal functional dynamics, essentially ‘resetting’ the dysfunctional circuits. This has powerful implications for how we conceptualize addiction at a systems neuroscience level—as a disorder amenable to circuit-level intervention, rather than purely chemical imbalance.
This novel approach aligns with a growing movement in psychiatry and neurology to leverage neuromodulation technologies such as TMS, transcranial direct current stimulation (tDCS), and deep brain stimulation (DBS) for the treatment of neuropsychiatric disorders. Previously established indications for TMS in depression and obsessive-compulsive disorder hint at its versatility, but its application in addiction medicine is still emergent. This study importantly validates the feasibility and efficacy of targeting frontocingulate pathways in a well-defined substance use disorder population.
The clinical translation of these findings could be transformative. Unlike pharmacological agents that require daily administration and carry risks of side effects or dependence, TMS offers a non-pharmacological, non-invasive intervention. Furthermore, identifying biomarkers such as aMCC activation patterns can refine patient selection and track treatment response, ushering in an era of personalized addiction medicine. These advances could ultimately reduce relapse rates and improve quality of life for millions suffering from opioid addiction.
However, the study authors caution that larger randomized controlled trials are essential to corroborate these preliminary outcomes and optimize stimulation parameters, including frequency, intensity, and duration of TMS sessions. The heterogeneity inherent to addiction—spanning differences in drug type, duration of use, comorbid psychiatric conditions, and individual neurobiology—necessitates flexible and adaptive therapeutic frameworks. Additionally, combining TMS with behavioral therapies or pharmacological agents could potentiate treatment efficacy, an avenue ripe for exploration.
On a neuroscientific front, this research enriches our understanding of the complex interplay between cognitive control networks and reward systems in addiction pathology. The aMCC, often underappreciated compared to classic reward centers like the ventral striatum or orbitofrontal cortex, emerges as a critical node whose dysfunction embodies the diminished reward learning and motivational deficits driving compulsive drug use. It also prompts further inquiry into whether similar neural mechanisms exist in other forms of addiction, such as stimulants or alcohol, thus broadening therapeutic horizons.
Technically, the study employed rigorous multimodal imaging paradigms combining fMRI with electrophysiological monitoring during TMS sessions, ensuring precise mapping of neural responses and stimulation effects. Advanced analytic approaches dissected signal changes at a fine temporal and spatial resolution, allowing for robust interpretation of frontocingulate circuit dynamics. This methodological sophistication strengthens confidence in the reported neurobiological mechanisms and their clinical relevance.
Moreover, the safety profile of prefrontal TMS was favorable, with minimal adverse events reported, underscoring its suitability for potential widespread clinical use. The non-invasive nature of TMS also sidesteps many obstacles associated with invasive neuromodulation strategies, such as DBS, including surgical risks and long-term device management. Accessibility and patient tolerance further support its integration into standard addiction treatment regimens.
As the opioid epidemic continues to ravage communities globally, fueled by potent synthetic opioids and limited treatment options, innovative interventions are desperately needed. This study provides hope that harnessing the plasticity of brain networks through technologies like TMS can heal the disrupted reward systems at the heart of addiction. It champions a conceptual shift from symptom management toward functional brain restoration—a paradigm that promises enduring recovery and resilience.
In conclusion, the normalization of anterior midcingulate cortex responses to reward in opioid users by prefrontal transcranial magnetic stimulation represents a seminal step forward in addiction neuroscience and therapy. By bridging molecular, circuit, and behavioral domains, this research pioneers a translational approach that can reshape therapeutic landscapes. Continued investigation into the optimization, integration, and long-term outcomes of TMS for OUD has the potential to revolutionize how we confront and conquer one of modern medicine’s most intractable challenges.
Subject of Research: Neural mechanisms underlying opioid addiction and modulation of reward processing via prefrontal transcranial magnetic stimulation
Article Title: Blunted anterior midcingulate response to reward in opioid users is normalized by prefrontal transcranial magnetic stimulation
Article References:
Biernacki, K., Goldstein, R.Z., Güth, M.R. et al. Blunted anterior midcingulate response to reward in opioid users is normalized by prefrontal transcranial magnetic stimulation. Transl Psychiatry 15, 340 (2025). https://doi.org/10.1038/s41398-025-03569-z
Image Credits: AI Generated