A groundbreaking study led by researchers at the University of Iowa has uncovered a striking new structural configuration for the DNA repair protein RAD52, specifically when it interacts with DNA in dividing cells. The implications of this discovery are significant, as RAD52 is a critical player in cellular mechanisms that repair DNA, particularly in cancer cells that exhibit deficiencies in conventional DNA repair pathways. The findings not only shed light on RAD52’s fundamental role in maintaining genomic integrity but also pave the way for the development of innovative anti-cancer therapies targeting this protein.
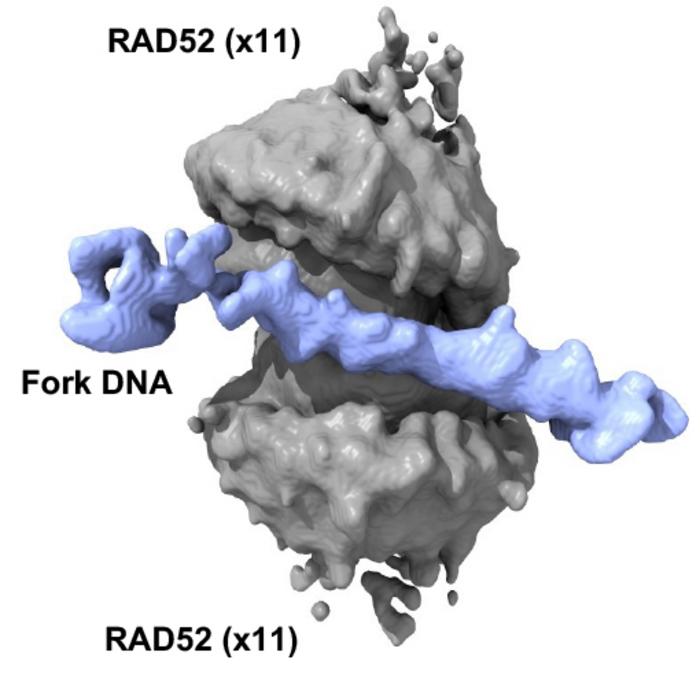
The research, published on April 2, 2025, in the esteemed journal Nature, presents an intricate picture of how RAD52 operates at the molecular level. Professor Maria Spies, a biochemist and molecular biologist at the University of Iowa, has detailed the unexpected dual-ring structure of RAD52 that emerges during its interaction with DNA. This unique configuration plays a crucial role in protecting the integrity of DNA as replication processes unfold, particularly when those processes are compromised by cellular stresses or deficiencies in DNA repair.
RAD52 has garnered attention as a promising target for cancer therapies, especially for malignancies stemming from genetic vulnerabilities such as BRCA1 and BRCA2 mutations. Traditional cancer treatments often overlook the need for targeted approaches that differentiate between healthy cells and those exhibiting dysregulated growth due to genetic aberrations. The research team found that while RAD52 is dispensable for the survival of healthy, non-cancerous cells, it becomes essential in cancer cells, which tend to exploit alternative mechanisms for DNA repair.
The methodology employed in this pioneering study hinged on advanced imaging techniques, specifically cryogenic electron microscopy (CryoEM). This state-of-the-art approach enabled the researchers to visualize the RAD52-DNA complex with unprecedented detail. The study revealed that RAD52 forms a remarkable spool-like structure comprising two concentric rings, each containing eleven RAD52 monomers. Furthermore, this configuration engages all three arms of a DNA replication fork, effectively stabilizing the structure and preventing degradation—crucial for cells that must complete DNA replication under unideal conditions.
Another critical aspect addressed in this research is the dynamic nature of the interactions between RAD52 and DNA. The findings indicate that these interactions are not static; rather, they involve a series of complex molecular transactions that regulate DNA repair processes. Understanding these dynamics is pivotal for designing small molecules that can inhibit RAD52’s function without adversely affecting the normal cellular functions in healthy tissues.
Previous studies have established RAD52’s protective role in salvaging stalled DNA replication forks, a feature particularly leveraged by cancer cells to survive and proliferate despite DNA damage. The implications of targeting RAD52-based therapies are substantial, as blocking this protein could selectively trigger apoptosis in cancer cells that are heavily reliant on its function for survival.
Moreover, the potential therapeutic application of RAD52 inhibitors is bolstered by prior evidence indicating that such inhibitors can effectively eliminate cancerous cells while mitigating toxic side effects associated with traditional chemotherapies. This characteristic aligns RAD52 inhibition with the concerted use of current therapies like PARP inhibitors, which address the specific vulnerabilities of BRCA1 and BRCA2 deficient cancers.
To consolidate the transition from research findings into clinical applications, the insights gained from this study are invaluable. The dual-ring architecture unveiled by the researchers holds promise for identifying specific regions of RAD52 to target for drug development. This precision could enhance the effectiveness of existing therapies as well as ensure fewer off-target effects in healthy cells, potentially leading to more personalized cancer treatment options.
What stands out about this research is the multidisciplinary collaboration reflected in the team’s efforts. The partnership between the University of Iowa scientists and experts from the Istituto Superiore di Sanità in Rome illustrates the importance of a collective approach in tackling complex biomedical questions. This collaboration underscores the global nature of scientific inquiry and the necessity of pooling expertise to achieve meaningful breakthroughs.
As the field of cancer research advances, the continued exploration of RAD52 is likely to yield further insights into its multifaceted role in both cancer biology and clinical therapeutics. The structural revelations provided by this study not only deepen our understanding of RAD52 but also highlight the intricate balance between DNA repair and cancer cell survival—a relationship that remains a cornerstone of cancer biology and treatment strategies.
Moving forward, the research team, including co-leads Masayoshi Honda and Mortezaali Razzaghi from the Spies lab, aims to refine their small-molecule inhibitors of RAD52. By improving the specificity and efficacy of these compounds, they hope to harness the full potential of RAD52 as a drug target, contributing to the next generation of anti-cancer therapies that can make a meaningful difference in patient outcomes.
In summary, the discovery of the dual-ring structure of RAD52 as it engages DNA represents a significant advancement in our understanding of DNA repair mechanisms in cancer cells. The ongoing research efforts facilitated by this foundational study hold considerable promise, not just for enhancing our comprehension of RAD52’s role in cellular biology, but also for translating this knowledge into innovative therapeutic strategies that could revolutionize the way we approach cancer treatment.
Subject of Research: Cells
Article Title: The RAD52 double-ring remodels replication forks restricting fork reversal
News Publication Date: 2-Apr-2025
Web References: Nature
References: Nature
Image Credits: Maria Spies, PhD, University of Iowa Health Care
Keywords: RAD52, DNA repair, cancer therapy, cryogenic electron microscopy, molecular biology, drug development