WHAT:
NIAID research has led to the availability of a new over-the-counter topical eczema probiotic. The probiotic is based on the discovery by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, that bacteria present on healthy skin called Roseomonas mucosa can safely relieve eczema symptoms in adults and children. R. mucosa-based topical interventions could simplify or complement current eczema management, when used in consultation with an individual’s healthcare provider. A milestone for eczema sufferers, the availability of an R. mucosa-based probiotic is the result of seven years of scientific discovery and research in NIAID’s Laboratory of Clinical Immunology and Microbiology (LCIM).

Credit: NIAID
WHAT:
NIAID research has led to the availability of a new over-the-counter topical eczema probiotic. The probiotic is based on the discovery by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, that bacteria present on healthy skin called Roseomonas mucosa can safely relieve eczema symptoms in adults and children. R. mucosa-based topical interventions could simplify or complement current eczema management, when used in consultation with an individual’s healthcare provider. A milestone for eczema sufferers, the availability of an R. mucosa-based probiotic is the result of seven years of scientific discovery and research in NIAID’s Laboratory of Clinical Immunology and Microbiology (LCIM).
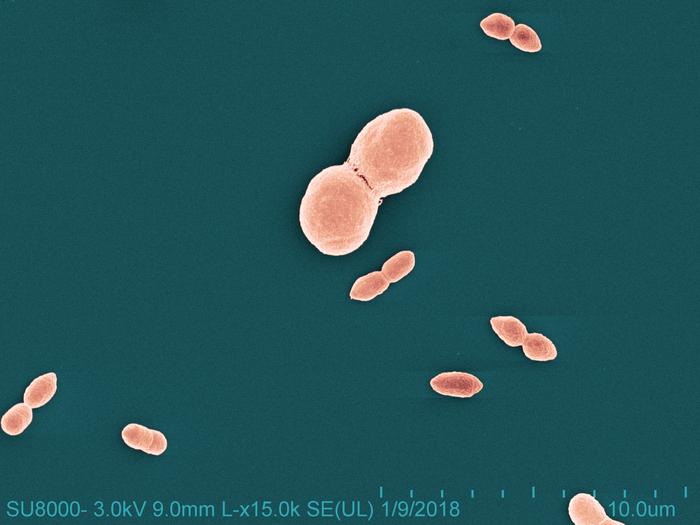
Eczema—also known as atopic dermatitis—is a chronic inflammatory skin condition that affects approximately 20% of children and 10% of adults worldwide. The condition is characterized by dry, itchy skin that can compromise the skin’s barrier, which functions to retain moisture and keep out allergens. This can make people with eczema more vulnerable to bacterial, viral and fungal skin infections. R. mucosa is a commensal bacterium, meaning it occurs naturally as part of a typical skin microbiome. Individuals with eczema experience imbalances in the microbiome and are deficient in certain skin lipids (oils). NIAID researchers demonstrated that R. mucosa can help restore those lipids.
Scientists led by Ian Myles, M.D., M.P.H., chief of the LCIM Epithelial Research Unit, found specific strains of R. mucosa reduced eczema-related skin inflammation and enhanced the skin’s natural barrier function in both adults and children. To arrive at this finding, Dr. Myles and colleagues spearheaded a spectrum of translational research on R. mucosa. They isolated and cultured R. mucosa in the laboratory, conducted preclinical (laboratory/animal) and clinical (human) studies, and made the bacteria available for commercial, non-therapeutic development. The R. mucosa-based probiotic released this week is formulated by Skinesa and called Defensin.
In Phase 1/2 open-label and Phase 2 blinded, placebo-controlled clinical studies, most people experienced greater than 75% improvement in eczema severity following application of R. mucosa. Improvement was seen on all treated skin sites, including the inner elbows, inner knees, hands, trunk and neck. The researchers also observed improvement in skin barrier function. Additionally, most participants needed fewer corticosteroids to manage their eczema, experienced less itching, and reported a better quality of life following R. mucosa therapy. These benefits persisted after treatment ended: therapeutic R. mucosa strains remained on the skin for up to eight months in study participants who were observed for that duration.
To expand the potential use of R. mucosa, NIAID will conduct an additional clinical trial to generate further evidence on its efficacy in reducing eczema symptoms. Those data could form the basis of an application to the Food and Drug Administration to enable the product to be regulated as a nonprescription drug and made accessible to a broader population of people with eczema. Study results are expected in 2024.
WHO:
Ian Myles, M.D., M.P.H, chief of the Epithelial Research Unit in NIAID’s Laboratory of Clinical Immunology and Microbiology, is available to comment.
CONTACT:
To schedule interviews, please contact the NIAID News & Science Writing Branch, (301) 402-1663, NIAIDNews@niaid.nih.gov.
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit
NIH…Turning Discovery Into Health®