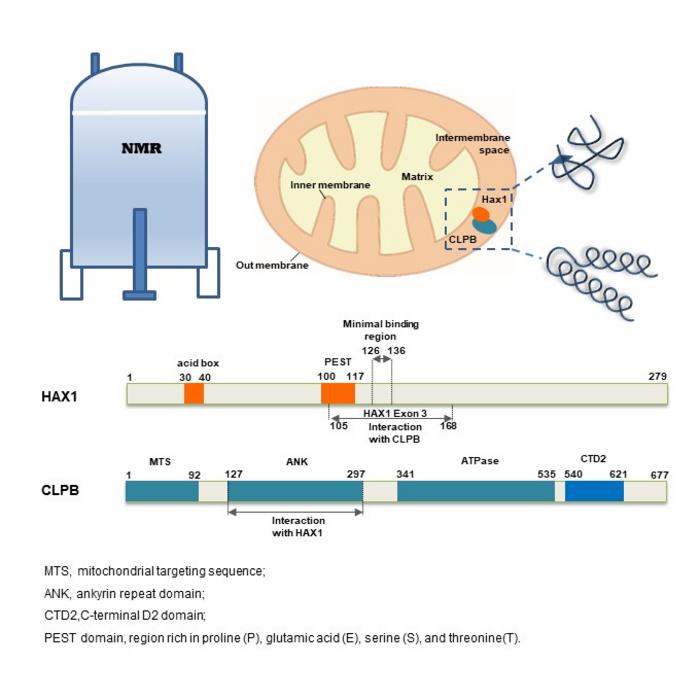
A groundbreaking exploration in the field of mitochondrial research has emerged from a recent study published in the prestigious journal Magnetic Resonance Letters. This innovative work uncovers the intricate interaction mechanism between two vital mitochondrial proteins, HAX1 and CLPB, offering insights that could reshape our understanding of their roles in cellular functions and disease pathways. The research, conducted by a team based in Hefei, China, employs an array of advanced biophysical techniques to dissect the binding affinity and structural dynamics of these proteins, thus bridging an important knowledge gap in mitochondrial biology.
HAX1, known as HCLS1-associated protein X-1, is primarily associated with critical biological processes such as apoptosis, mRNA processing, and the regulation of calcium homeostasis. Despite its significance, the intrinsically disordered nature of HAX1 has historically complicated efforts to elucidate its molecular mechanisms. In contrast, CLPB is a mitochondrial protein equipped with ATPase activity, which plays a crucial role in modulating deaggregase activity through a complex dynamic assembly process involving conformational transitions. This unique structural dichotomy between HAX1 and CLPB highlights their functional interdependence and suggests a potential avenue for therapeutic intervention.
The research employs cutting-edge nuclear magnetic resonance (NMR) technology to assess the binding dynamics between HAX1 and CLPB with remarkable precision. Findings indicate that the multi-protein complex formed by these two proteins has a low dissociation constant (Kd), specifically in the low micromolar range, signifying a stable and efficient interaction. Such a robust binding affinity not only emphasizes the importance of the HAX1-CLPB interaction but also provides a foundation for understanding how these proteins may cooperate in regulatory functions within the mitochondrion.
Further experimental validation through sophisticated techniques like molecular exclusion chromatography and dynamic light scattering serves to reinforce the findings. These methods confirm that the assembly of the HAX1-CLPB complex is primarily driven by specific interactions between the disordered region of HAX1 and the structured helical domain of CLPB. This specificity underscores the significance of structural complementarity in determining functional synergy within protein complexes, an insight that could direct future studies on protein interactions in various biological contexts.
As the insights from this study continue to unfold, the implications for therapeutic strategies become increasingly evident. Prof. Junfeng Wang, the corresponding author of the study, articulates a vision for the future of this research, emphasizing the critical need to explore the energetic landscapes and dynamic behaviors of the HAX1-CLPB complex. By facilitating the resolution of the three-dimensional structure of this complex through advanced imaging techniques such as cryo-electron microscopy, researchers hope to uncover the molecular intricacies that underpin disease-related pathways.
The aberrant expression of HAX1 has been linked to numerous pathologies, including impaired angiogenesis and immune cell apoptosis in diseases such as nasopharyngeal carcinoma metastasis. On the other hand, mutations in CLPB are known to correlate with genetic disorders characterized by mitochondrial dysfunctions, including mitochondrial encephalomyopathy. Hence, the elucidation of the interaction framework between HAX1 and CLPB not only sheds light on their biological significance but also opens new avenues for targeted therapeutic strategies aimed at addressing the underlying causes of these complex diseases.
This research embodies a significant step forward in mitochondrial protein interaction studies, illustrating how fundamental biophysical methodologies can yield insights with profound clinical relevance. As researchers continue to dissect the complexities of mitochondrial biology, the interplay between HAX1 and CLPB may serve as a model for studying other vital protein interactions within this organelle, thereby enriching our understanding of mitochondrial behavior in health and disease.
In an era where mitochondrial dysfunction is increasingly implicated in a range of diseases, the importance of insights like those provided by this study cannot be overstated. By exploring the cooperative interactions between mitochondrial proteins such as HAX1 and CLPB, researchers are not only enhancing our grasp of mitochondrial dynamics but also paving the way for innovative therapeutic approaches. The future of research in this domain appears promising, with potential breakthroughs on the horizon that could significantly impact the treatment of mitochondrial and related diseases.
The momentum generated by this research presents a clarion call for continued exploration of protein interactions within mitochondria. As we stand at the crossroads of mitochondrial biology and therapeutic innovation, the findings of the ongoing studies on HAX1 and CLPB promise to inspire a wave of new research aimed at understanding the multifaceted roles of mitochondrial proteins. These proteins are now recognized not merely as functional entities but as critical players in the cellular dialogue that governs life and death at the cellular level.
Moreover, as this field of research flourishes, collaboration among scientists, clinicians, and molecular biologists will be crucial in translating these discoveries into real-world applications. The hope is that a comprehensive understanding of mitochondrial protein interactions will lead to novel therapeutic interventions that can effectively mitigate the consequences of mitochondrial dysfunction across various diseases, ultimately improving patient outcomes.
Through the rigorous investigation of mitochondrial proteins such as HAX1 and CLPB, the scientific community is one step closer to demystifying the complexities of cellular machinery. As this research spurs further inquiries and technological advancements, the future looks bright, heralding a new era in our understanding of mitochondria and their essential roles in health and disease.
Subject of Research: Protein interactions in mitochondrial biology
Article Title: Biophysical and NMR analysis reveals binding affinity between HAX1 and CLPB proteins
News Publication Date: October 2023
Web References: http://dx.doi.org/10.1016/j.mrl.2024.200141
References: Not applicable
Image Credits: JING YANG
Keywords: Mitochondrial proteins, HAX1, CLPB, NMR, biophysical techniques, protein interactions, therapeutic strategies, mitochondrial dysfunction.