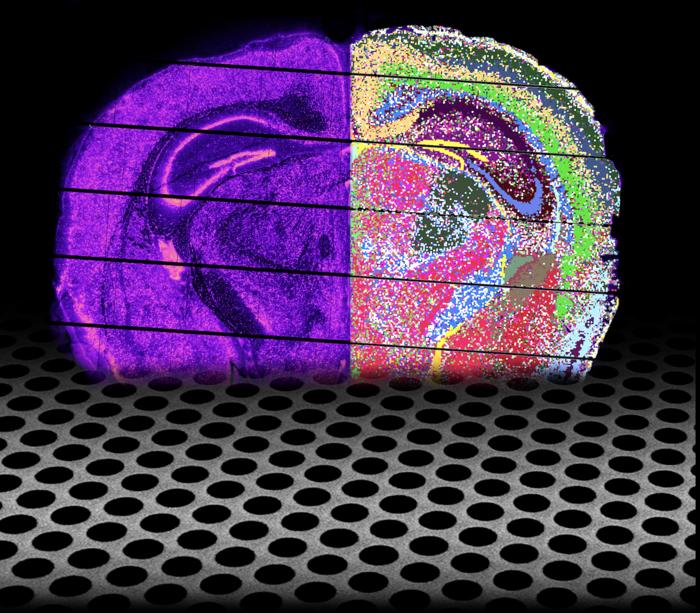
Leuven, 6 August 2024 – A team of researchers from the lab of Prof. Stein Aerts (VIB-KU Leuven) presents Nova-ST, a new spatial transcriptomics technique that promises to transform gene expression profiling in tissue samples. Nova-ST will make large-scale, high-resolution spatial tissue analysis more accessible and affordable, offering significant benefits for researchers. The research was published in Cell Reports Methods.

Credit: COPYRIGHT: VIB
Leuven, 6 August 2024 – A team of researchers from the lab of Prof. Stein Aerts (VIB-KU Leuven) presents Nova-ST, a new spatial transcriptomics technique that promises to transform gene expression profiling in tissue samples. Nova-ST will make large-scale, high-resolution spatial tissue analysis more accessible and affordable, offering significant benefits for researchers. The research was published in Cell Reports Methods.
Transcriptomics is the study of gene expression in a cell or a population of cells, but it usually does not include spatial information about where those genes were active. This hurdle limited our understanding of complex biological processes that rely on specific gene activity patterns within tissues. Thankfully, spatial transcriptomics has emerged as a powerful tool, allowing scientists to map gene expression across a tissue section with a spatial context. However, existing techniques often suffer from high costs, limited resolution, or compatibility issues.
Enter Nova-ST, a novel open-source spatial transcriptomics workflow developed by the lab of Prof. Stein Aerts and the Single-cell Microfluidics and Bioinformatics expertise units at VIB.AI and the VIB-KU Leuven Center for Brain & Disease Research. Nova-ST overcomes these limitations with its innovative approach, offering greater affordability, impressive resolution, and versatility.
Under the hood
At the heart of Nova-ST lies a clever adaptation of Illumina NovaSeq 6000 S4 or the new generation Novaseq X sequencing flow cells, commonly used for large-scale DNA sequencing. These flow cells contain a dense nano-patterned surface riddled with tiny, randomly barcoded nanowells arranged in a hexagonal lattice. Each well acts as a capture site for mRNA molecules from a specific location within the tissue sample. This dense nano-patterned surface allows Nova-ST to achieve high spatial resolution, potentially capturing the footprint of single cells.
“We then use these capture sites to snag mRNA molecules while preserving their spatial coordinates,” explains Dr. Suresh Poovathingal, who led the experimental development and optimization. ”Sequencing these captured mRNA molecules reveals the gene expression profile for each capture site. By piecing together this information, we can reconstruct a detailed map of gene activity across the entire tissue section.”
Advantages
The new platform boasts several key advantages. Firstly, it is cost-effective. By leveraging readily available Illumina flow cells and employing a novel chip-cutting technique, multiple Nova-ST chips can be created from a single flow cell, significantly reducing costs compared to existing methods. Secondly, the dense nano-patterned surface allows Nova-ST to achieve high spatial resolution, potentially capturing gene expression at the single-cell level. Thirdly, Nova-ST is compatible with various tissue types, making it a versatile tool for studying diverse biological systems. Additionally, the compatibility with next-generation Illumina flow cells suggests that Nova-ST can benefit from advancements in sequencing technology.
“Importantly, Nova-ST’s open-source nature makes the protocol accessible to a wider range of researchers and allows for further customization,” Dr. Kristofer Davie, who led the data analysis, notes, “Our workflow is designed to be user-friendly and adaptable, ensuring that researchers can tailor the technique to their specific needs.”
Impact
Nova-ST is the latest example of broader efforts within the spatial transcriptomics research community to democratize access and build platforms that advance a wide range of biomedical research, including Seq-Scope and its recent variants developed at the University of Michigan, as well as the recently published Open-ST platform developed by scientists at Max Delbrück Center in Germany.
Stein Aerts’ lab and the expertise units are already applying Nova-ST to advance their colleagues’ research in neurodegeneration and cancer biology. For example, they processed muscle samples for the Sandrine Da Cruz lab (VIB-KU Leuven) to study the effects of neurodegenerative diseases on neuromuscular junctions. Additionally, they are working with the Diether Lambrechts lab (VIB-KU Leuven) to expand the Nova-ST platform. This expansion will allow the simultaneous spatial analysis of immune cell receptors and gene expression, enabling the study of immune cell distribution in tumors undergoing immunotherapy. These collaborations highlight Nova-ST’s practical applications and its potential to impact various fields.
Prof. Stein Aerts emphasizes, “Nova-ST is a game-changer for research across multiple fields, from cancer biology to plant biology. By making this platform open source, we aim to empower scientists worldwide to explore and innovate.”
The detailed experimental protocols are available here and here. The computational pipeline is available on GitHub, where the entire scientific community can make use of it for implementing Nova-ST. Additional details can be found at nova-st.aertslab.org.
—
Publication
Nova-ST: Nano-Patterned Ultra-Dense platform for spatial transcriptomics. Poovathingal, Davie, et al. Cell Reports Methods, 2024. DOI: xxx
Funding
The research (team) was supported by an ERC Advanced Grant and Aligning Science Across Parkinson’s through the Michael J. Fox Foundation for Parkinson’s Research.
Journal
Cell Reports Methods
Method of Research
Computational simulation/modeling
Subject of Research
Not applicable
Article Title
Nova-ST: Nano-Patterned Ultra-Dense platform for spatial transcriptomics
Article Publication Date
6-Aug-2024