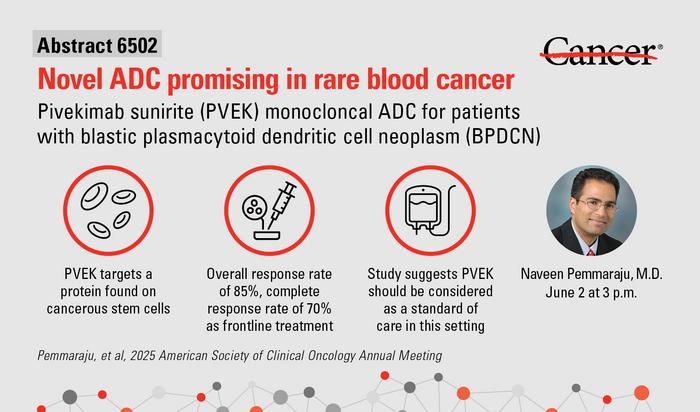
In a groundbreaking development in the treatment of rare hematologic malignancies, researchers have unveiled promising results for a novel antibody-drug conjugate (ADC) named pivekimab sunirine (PVEK) in patients diagnosed with blastic plasmacytoid dendritic cell neoplasm (BPDCN). BPDCN is an exceedingly aggressive and uncommon blood cancer characterized by malignant involvement of the bone marrow, skin, and occasionally lymph nodes. The therapeutic landscape for BPDCN has historically been limited, underscoring the urgent need for innovative and effective frontline therapies. The recent clinical trial findings, presented at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, spotlight PVEK’s potential to become a transformative treatment option.
PVEK, a next-generation ADC, functions through precision targeting of CD123, a receptor highly expressed on BPDCN cells. By coupling a monoclonal antibody to a potent cytotoxic agent via a chemical linker, PVEK selectively delivers its therapeutic payload to malignant cells, minimizing systemic toxicity. This mode of action exemplifies the paradigm shift in oncology toward highly specific, biomarker-driven treatments, enabling enhanced efficacy alongside improved safety profiles. The CADENZA study, a multi-center Phase I/II trial, evaluated PVEK in both treatment-naïve and relapsed/refractory BPDCN cohorts, providing robust data indicative of significant clinical benefit.
Within the trial’s frontline treatment arm, 33 newly diagnosed BPDCN patients received intravenous administration of PVEK on a 21-day cycle regimen. The results demonstrated an overall response rate of 85%, a substantial proportion of which—70%—achieved complete remission. These findings signify a noteworthy advancement given BPDCN’s historical resistance to conventional chemotherapy and poor prognosis. Moreover, the median overall survival across this cohort was recorded at 16.6 months, a remarkable figure reflecting PVEK’s potential to extend patient survival and enhance quality of life.
The compelling efficacy data of PVEK are complemented by its safety profile. The most commonly observed adverse event was peripheral edema, which was reversible and clinically manageable, underscoring the treatment’s tolerability in an often frail patient population. This contrast to the current standard of care, tagraxofusp-erzs, which, while targeting the same CD123 receptor, carries a distinct toxicity spectrum including capillary leak syndrome, highlights the therapeutic advantage that PVEK may offer.
Delving deeper into PVEK’s mechanism, the antibody component specifically recognizes the CD123 antigen, highly expressed on BPDCN tumor cells, facilitating internalization. Once internalized, the conjugated payload induces targeted cytotoxicity through DNA damage or disruption of microtubule dynamics, depending on the payload’s nature. This precise delivery system mitigates collateral damage to non-malignant cells, a limitation inherent to conventional chemotherapeutics and some earlier ADCs.
The CADENZA trial incorporated 84 adult participants across multiple global centers, including patients with relapsed or refractory disease who had undergone one to three prior lines of therapy. Such inclusion criteria emphasize the trial’s comprehensive approach to evaluating PVEK’s efficacy across varied disease settings. While the newly diagnosed cohort yielded the most striking response rates, ongoing analyses will further elucidate outcomes in relapsed/refractory populations, potentially expanding PVEK’s therapeutic applicability.
Importantly, the success of this ADC aligns with a broader oncology trend embracing the concept of precision medicine. By exploiting tumor-specific surface markers, ADCs like PVEK herald an era where targeted cytotoxic delivery can surmount the challenges posed by heterogeneity and drug resistance characteristic of malignancies like BPDCN. Additionally, the modular nature of ADC design allows for strategic combination with other immunomodulatory or cytotoxic agents, an avenue currently under consideration for future clinical exploration.
The development of PVEK is also emblematic of the synergy between academic research and pharmaceutical innovation. Funded by AbbVie, the clinical study reflects a collaborative effort involving expert clinicians and scientists led by Dr. Naveen Pemmaraju and Dr. Naval Daver from The University of Texas MD Anderson Cancer Center, institutions renowned for their pioneering work in leukemia research. Their leadership underscores the critical role of specialized cancer centers in driving forward translational research that bridges laboratory insights to patient care.
A historical perspective underscores the significance of advances such as PVEK. Prior to the advent of targeted therapies, BPDCN management predominantly consisted of non-specific cytotoxic chemotherapy with limited efficacy and substantial toxicity. The approval of tagraxofusp-erzs in 2018 represented a milestone for targeting CD123; however, unmet clinical needs persist, particularly in terms of durable responses and safety. PVEK’s emergence as a potent ADC with a promising therapeutic index offers renewed hope to patients who face limited options.
The favorable safety and efficacy profile of PVEK observed thus far opens the door for future investigations into combinatorial approaches. Preclinical models suggest that ADCs targeting CD123 may synergize with agents such as immune checkpoint inhibitors, hypomethylating agents, or novel small molecules targeting intracellular oncogenic pathways. Such multidisciplinary strategies could potentiate anti-tumor responses while circumventing mechanisms of resistance that often emerge during monotherapy.
In conclusion, the data presented from the Phase I/II CADENZA trial firmly position pivekimab sunirine as a breakthrough advancement in the management of BPDCN. High overall and complete response rates, coupled with manageable toxicity, highlight PVEK’s potential to redefine standard-of-care treatments for this rare and aggressive malignancy. Ongoing studies and future expansions into combination regimens may further optimize therapeutic outcomes. This advance exemplifies the power of antigen-directed therapies to transform the outlook for patients with historically challenging hematological cancers.
Subject of Research: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) and targeted antibody-drug conjugate therapy
Article Title: [Not provided explicitly]
News Publication Date: June 2, 2025
Web References:
– ASCO Annual Meeting Abstract: https://meetings.asco.org/abstracts-presentations/244202
– MD Anderson Cancer Center: https://www.mdanderson.org/
– ASCO Annual Meeting: https://www.asco.org/annual-meeting
Image Credits: The University of Texas MD Anderson Cancer Center
Keywords: Blood cancer, Blastic plasmacytoid dendritic cell neoplasm, Antibody-drug conjugate, Pivekimab sunirine, CD123, Targeted therapy, Hematologic malignancy, Oncology, Clinical trial, ASCO 2025