In a groundbreaking study published in Cell Death Discovery, researchers have unveiled a promising new avenue for treating PAX3–FOXO1 rhabdomyosarcoma, an aggressive pediatric cancer notorious for its poor prognosis and resistance to conventional therapies. The research focuses on the inhibition of a critical post-translational modification process known as neddylation and its profound impact on tumor dynamics and radiosensitivity.
Rhabdomyosarcoma, particularly the variant driven by the PAX3–FOXO1 fusion gene, represents a formidable challenge in oncology due to its enhanced proliferative capacity and survival mechanisms. The PAX3–FOXO1 fusion protein acts as a potent oncogenic driver, altering gene expression and fostering an environment conducive to tumor progression. Targeting pathways that regulate this fusion protein or its downstream effects is therefore a priority in the development of effective therapies.
Neddylation is a ubiquitin-like modification that attaches the small protein NEDD8 to target substrates, fundamentally influencing protein stability, function, and interaction. This process, tightly regulated under physiological conditions, is co-opted by cancer cells to sustain malignant behaviors, including unchecked growth and evasion of apoptosis. By inhibiting neddylation, cancer cells lose a critical regulatory mechanism, rendering them vulnerable to DNA damage and therapeutic intervention.
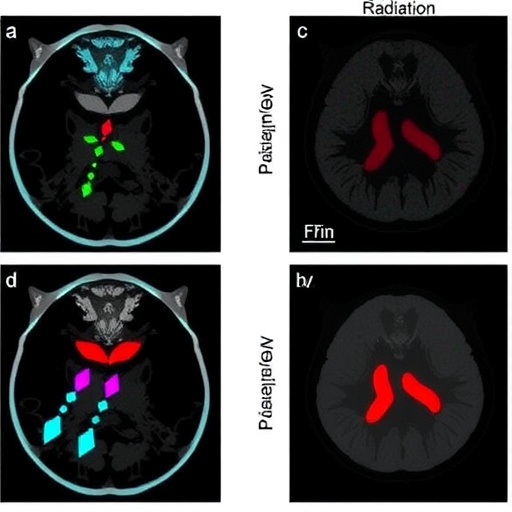
The current study employed pharmacological agents to disrupt the neddylation cascade in models of PAX3–FOXO1 rhabdomyosarcoma, revealing an accumulation of DNA double-strand breaks (DSBs). These breaks represent the most lethal form of DNA damage, challenging the integrity of the cancer genome and precipitating cellular demise. Intriguingly, the induction of DSBs in these tumors was accompanied by a marked deceleration in tumor growth when studied in vivo, underscoring the potential clinical relevance of neddylation inhibition.
Moreover, the researchers uncovered a significant enhancement in the tumor cells’ sensitivity to ionizing radiation following neddylation blockade. Radiosensitivity is a crucial factor in cancer treatment, and many tumors, including PAX3–FOXO1 rhabdomyosarcoma, display inherent or acquired resistance to radiation therapy. By promoting radiosensitivity, neddylation inhibitors could synergize with existing radiotherapy regimens, amplifying their efficacy and potentially leading to improved patient outcomes.
Mechanistically, the study delved into the molecular aftermath of neddylation inhibition. The accumulation of DSBs was accompanied by impaired DNA damage repair pathways, particularly homologous recombination and non-homologous end joining. Key proteins involved in these pathways failed to localize correctly or function efficiently without neddylation, disrupting the cancer cell’s ability to mend lethal DNA lesions.
This disruption of repair machinery not only explains the buildup of DNA damage but also provides insight into why cancer cells become exquisitely sensitive to radiotherapy under these conditions. Radiation itself induces DNA breaks; therefore, cells unable to repair such damage succumb more readily, an effect that can be exploited therapeutically.
Importantly, the study extended beyond in vitro observations, demonstrating that treatment with neddylation inhibitors markedly impaired tumor growth in mouse xenograft models bearing PAX3–FOXO1 rhabdomyosarcoma tumors. These findings validate the translational potential of targeting neddylation, moving the concept closer to clinical application.
In addition to the direct antitumor effects, the research highlighted the specificity of neddylation inhibition’s impact on malignant cells. Normal cells displayed relative resilience to these inhibitors, suggesting a therapeutic window that could mitigate systemic toxicity—a major hurdle in pediatric oncology drug development.
Further examination revealed that the PAX3–FOXO1 fusion protein itself might be intricately linked to the heightened reliance on neddylation in this rhabdomyosarcoma subtype. This fusion oncoprotein potentially drives pathways that increase protein turnover and stress responses requiring neddylation, selectively sensitizing these cancer cells to its inhibition.
The study’s implications extend beyond rhabdomyosarcoma, as neddylation has been implicated in the pathogenesis and progression of various cancers. The successful demonstration of radiosensitizing effects alongside tumor growth suppression opens avenues for combination therapies that might overcome resistance mechanisms prevalent in multiple malignancies.
Notably, this research complements emerging trends in precision oncology, where understanding tumor-specific vulnerabilities guides therapeutic strategies. Targeting a fundamental protein modification pathway harnesses a novel mechanism that could integrate with genetic and epigenetic targeting agents currently under investigation.
While the study is remarkable, it also paves the way for further investigations. Key questions remain about the long-term effects of neddylation inhibition, potential resistance mechanisms that tumors might develop, and optimal integration with existing chemotherapeutic and radiotherapeutic protocols.
Moreover, understanding the influence of neddylation inhibition on the tumor microenvironment, immune modulation, and systemic responses will be essential to fully realize the therapeutic potential and safety of this approach.
Clinical translation will require careful dose optimization and biomarker development to identify patients who might benefit most from neddylation-targeted therapies, especially considering the heterogeneity within rhabdomyosarcoma and other sarcomas.
Given the devastating prognosis for many children afflicted with PAX3–FOXO1 rhabdomyosarcoma, this innovative approach offers a beacon of hope. By exploiting a critical cellular process that cancer cells depend on, this strategy holds promise for more effective and less toxic treatments that could transform outcomes in pediatric oncology.
The exciting convergence of molecular biology, pharmacology, and clinical oncology in this study exemplifies the cutting edge of cancer research, bringing us closer to treatments that not only extend life but improve its quality for children worldwide.
As research into neddylation inhibitors proceeds, integration with other targeted agents, including immunotherapies and gene editing technologies, may yield even more powerful strategies against resistant and aggressive tumors.
In conclusion, the inhibition of neddylation emerges as a sophisticated mechanism that undermines tumor survival by triggering unrepaired DNA damage and sensitizing cancer cells to radiation, offering a novel therapeutic paradigm for combating PAX3–FOXO1 rhabdomyosarcoma and potentially other malignancies.
Subject of Research: Neddylation inhibition as a therapeutic strategy in PAX3–FOXO1 rhabdomyosarcoma, focusing on its role in inducing DNA double-strand breaks and enhancing radiosensitivity to suppress tumor growth.
Article Title: Neddylation inhibition induces DNA double-strand breaks, hampering tumor growth in vivo, and promotes radiosensitivity in PAX3–FOXO1 rhabdomyosarcoma.
Article References:
Aiello, F.A., D’Archivio, L., Attili, M. et al. Neddylation inhibition induces DNA double-strand breaks, hampering tumor growth in vivo, and promotes radiosensitivity in PAX3–FOXO1 rhabdomyosarcoma. Cell Death Discov. 11, 496 (2025). https://doi.org/10.1038/s41420-025-02787-0
Image Credits: AI Generated
DOI: 10.1038/s41420-025-02787-0 (Published 03 November 2025)