A groundbreaking discovery in the field of systems biology has emerged from a collaborative effort between researchers at the University of Potsdam and the Max Planck Institute of Molecular Plant Physiology in Golm. Their study introduces a transformative concept known as kinetic modules in biochemical networks, which has the potential to redefine our understanding of how cellular biochemical systems maintain function and stability amidst fluctuating environmental conditions. Published in the prestigious journal Science Advances on May 28, 2025, the research addresses a systems biology challenge that has perplexed scientists for over thirty years by elucidating the connection between the structural organization and dynamic behavior of biochemical reaction networks.
At the cellular level, biochemical networks operate as intricate information-processing systems, mediating critical functions such as signal transduction, metabolic conversions, and homeostatic regulation. These networks comprise myriad chemical reactions that convert substrates into essential biomolecules, thereby sustaining life processes. Traditionally, attempts to decipher these complex systems have relied heavily on structural analyses, which identify modules—discrete subsets of the network based on topology or connectivity. However, such structural modularity alone has only partially explained the dynamic robustness exhibited by cells, leaving a significant gap in understanding how biochemical systems reliably maintain metabolite concentrations in varying environments.
The new research pivots on the interplay between network structure and kinetic dynamics, focusing specifically on the coupling patterns of reaction rates. Kinetic modules, as defined by the team, are functional units emerging from these kinetic couplings rather than mere static network architecture. This insight enables the dissection of biochemical networks in a manner that incorporates both the physical connectivity of reactions and the quantitative dynamics governing their rates. The primary scientific question guiding this study was how these kinetic modules contribute to the concentration robustness of metabolites—a fundamental property that ensures cellular survival by preventing detrimental fluctuations in metabolite levels under changing environmental parameters.
Zoran Nikoloski, Professor of Bioinformatics at the University of Potsdam and a pivotal figure in the research, articulates the significance of kinetic modules: "By identifying functional units defined by their kinetic interdependence, we elucidate fundamental mechanisms that render metabolic concentrations robust. These stable concentration profiles are critical for cellular adaptability, and their loss underlies numerous pathological states." This assertion highlights the clinical and biotechnological ramifications of the findings, pointing to potential new avenues for therapeutic intervention and metabolic engineering based on the modular kinetic organization of biochemical systems.
The researchers systematically analyzed 34 comprehensive metabolic network models spanning 26 phylogenetically diverse organisms, encompassing central model species such as Arabidopsis thaliana, Escherichia coli, and Saccharomyces cerevisiae. Employing computational models that integrate stoichiometry, enzyme kinetics, and flux distributions, the team applied the kinetic module framework to dissect how subsets of reactions function cooperatively to stabilize metabolite concentrations. Unlike purely topological modules, kinetic modules revealed dynamic interdependencies that explain emergent properties observed experimentally but elusive to prior structural models. This approach represents a conceptual shift, grounding modularity not only in who connects to whom but in how their reaction velocities synchronize to preserve cellular homeostasis.
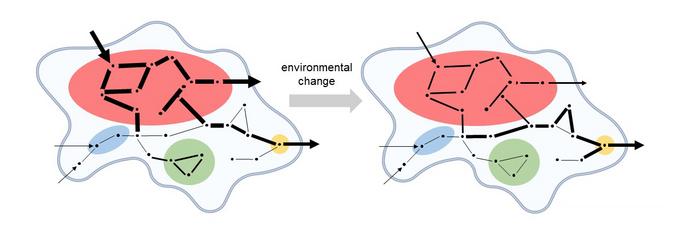
One of the most compelling results of the study lies in demonstrating that kinetic modules act as intrinsic sources of concentration robustness, effectively insulating metabolic outputs from perturbations. This mechanistic insight provides a quantifiable link between enzyme kinetics and systemic metabolic stability, clarifying how biochemical networks buffer fluctuations caused by environmental changes or genetic variations. The thickness of reaction pathways within kinetic modules, representing higher metabolic fluxes, illustrates the differentiated flow distribution that underlies module functionality, offering fresh interpretive frameworks for biochemical network dynamics.
Beyond the immediate biochemical significance, the introduction of kinetically defined modules opens new horizons for systems biology and synthetic biology. Automated kinetic module identification pipelines can facilitate a deeper understanding of the cross-talk between metabolic, signaling, and regulatory networks, potentially uncovering universal design principles that operate above mere structural constraints. By integrating kinetic module analysis, researchers and engineers could optimize metabolic pathways, improving yield and robustness in industrial biotechnology or devising novel therapeutic strategies that target specific dynamic modules to restore metabolic homeostasis in disease contexts.
The study also invites a reevaluation of evolutionary perspectives on biochemical networks. Kinetic modules may represent evolutionary conserved functional units shaped not only by genetic selection on structural motifs but also by the dynamic necessities of maintaining stable metabolite concentrations. This dynamic perspective provides a fertile ground for future research exploring how evolutionary pressures sculpt both the architecture and kinetic parameters of biochemical networks to achieve robustness and adaptability.
Complementing the theoretical and computational insights, the research includes visually striking illustrations that depict biochemical networks with colored kinetic modules and reaction flows represented by arrows of varying thickness. These visualizations encapsulate the complexity and elegance of metabolic interactions, highlighting how kinetic coupling orchestrates cellular metabolism in a modular fashion. By enabling intuitive comprehension of otherwise abstract dynamic relationships, these figures serve as valuable tools for education and further investigation.
The implications of this research extend well beyond academic circles. Understanding and manipulating kinetic modules could revolutionize precision medicine, as alterations in kinetic module stability may underpin metabolic dysregulation observed in diseases such as cancer, diabetes, and metabolic syndromes. The ability to pinpoint and modulate kinetic modules may pave the way for therapies that restore cellular robustness without broadly disrupting network functionality, thereby reducing side effects and increasing efficacy.
Furthermore, the researchers emphasize the versatility of their framework in diverse biological contexts. The methodology is applicable not only to well-characterized model organisms but also to emerging models and complex multicellular systems, offering a scalable framework for dissecting biochemical complexity across scales. This universality underscores the robustness of the kinetic module concept as a foundational principle within systems biology.
In conclusion, the introduction of kinetic modules as a bridging concept between biochemical network structure and dynamics represents a seminal advance in our understanding of cellular systems. By revealing how kinetic interdependencies shape concentration robustness, this work resolves a longstanding enigma in systems biology, opening new frontiers in research and application. The publication marks a critical milestone, announcing a paradigm shift in how scientists conceptualize and analyze the living cell’s biochemical circuitry.
Subject of Research: Not applicable
Article Title: Kinetic modules are sources of concentration robustness in biochemical networks
News Publication Date: 28-May-2025
Web References:
http://dx.doi.org/10.1126/sciadv.ads7269
References:
Langary et al., Science Advances 11, eads7269 (2025)
Image Credits:
Illustration: Zoran Nikoloski
Keywords:
Biochemistry