In the rapidly evolving field of neurogenetics, the advent of cutting-edge technologies has continuously unveiled intricate mechanisms underlying neurodegenerative disorders. A recent groundbreaking study published in Cell Death Discovery has brought to light crucial insights into how novel mutations in the aprataxin (APTX) gene may deeply impact neural development and DNA repair pathways. Researchers from an international consortium, led by Chen, Z., Huang, Y., and Yuan, Z., have demonstrated that induced pluripotent stem cells (iPSCs) harboring previously unidentified APTX mutations exhibit impaired neural differentiation, a phenomenon tightly coupled with the accumulation of DNA single-strand breaks. This discovery undoubtedly expands our understanding of the molecular pathology of ataxia-related disorders and other neurodegenerative diseases linked to DNA repair deficiencies.
The APTX gene encodes aprataxin, a protein essential for the resolution of abortive DNA ligation intermediates during single-strand break repair. Single-strand breaks (SSBs) are a common form of DNA damage that, if left unrepaired, can lead to deleterious consequences such as genomic instability and cell death. The precise role of aprataxin in neural differentiation has remained an area of intense investigation, particularly given its association with ataxia with oculomotor apraxia type 1 (AOA1), a rare neurodegenerative disorder characterized by motor coordination deficits. The innovative approach of utilizing iPSCs in this study provides a robust model system to dissect the cellular repercussions of APTX mutations in a human genetic background.
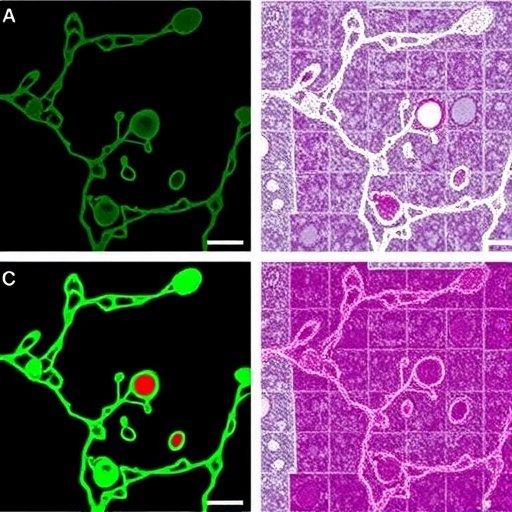
By generating iPSCs from patient-derived fibroblasts bearing novel APTX mutations, the investigators were able to closely mimic the endogenous cellular environment while observing the effects on neural lineage commitment. The study employed a suite of sophisticated techniques, including immunocytochemistry, comet assays, and next-generation sequencing, to quantify DNA damage and assess differentiation potential. Notably, cells carrying mutant APTX alleles exhibited a marked failure to progress from neural progenitors to mature neurons, accompanied by a significant increase in DNA single-strand breaks, as evidenced by elevated levels of γH2AX and other DNA damage markers.
These findings suggest a dual pathological mechanism wherein defective DNA repair not only compromises genomic integrity but also actively hinders neurogenesis. This dual threat highlights the intricate interplay between DNA damage response pathways and neuronal differentiation programs. The accumulation of unrepaired single-strand breaks was postulated to trigger aberrant activation of cellular stress responses, thereby impeding the tightly regulated transcriptional cascades essential for neural maturation. This mechanistic insight provides a compelling link between DNA repair deficiency and neurodevelopmental anomalies.
Furthermore, the study explored the downstream signaling pathways altered in mutant cells, revealing dysregulation of key genes implicated in neurogenesis and synaptic function. This dysregulation was correlated with impaired functional neuronal phenotypes, as mutant iPSC-derived neurons displayed altered electrophysiological properties in patch-clamp recordings. Such functional deficits aligned with the pathophysiological manifestations observed in patients harboring APTX mutations, thereby offering a potent translational bridge between molecular findings and clinical phenotypes.
The implications of this research are profound for the field of regenerative medicine and therapeutic development. Understanding the molecular underpinnings of impaired neural differentiation linked to DNA repair defects opens avenues for targeted interventions that could potentially restore neuronal function or prevent disease progression. Small molecule modulators of DNA repair enzymes or gene-editing strategies to correct pathogenic APTX mutations in patient-derived cells are among the promising future directions prompted by this study.
Moreover, this investigation underscores the utility of iPSC technology as a platform for modeling complex genetic diseases and screening therapeutic candidates. By recapitulating patient-specific genetic mutations within a controlled in vitro environment, researchers can gain unprecedented insights into disease mechanisms, paving the way for personalized medicine approaches. The integration of advanced genomic and cellular techniques, as demonstrated here, exemplifies the frontier of neurogenetic research.
The accumulation of DNA single-strand breaks as a hallmark of APTX mutations evokes parallels with other neurodegenerative disorders marked by genomic instability, suggesting a convergent disease mechanism. Conditions such as ataxia telangiectasia, Huntington’s disease, and certain forms of amyotrophic lateral sclerosis also exhibit impaired DNA repair capacity, reinforcing the concept that maintaining genome integrity is paramount for neuronal survival and function. This study adds a vital piece to the puzzle by delineating how defective repair not only triggers neurodegeneration but also disrupts the earliest steps of neural development.
Intriguingly, the authors report that attempts to pharmacologically bolster DNA repair pathways in mutant iPSC cultures partially rescued the differentiation defects, indicating that intervention at the level of DNA maintenance could hold therapeutic promise. The potential to ameliorate neurodevelopmental disruptions by modulating DNA repair enzymes suggests an innovative therapeutic axis distinct from conventional neuroprotective strategies. Future research will be critical to refine these approaches and assess efficacy in vivo.
On a broader spectrum, this research may extend beyond APTX-associated disorders to inform our understanding of aging and other neurological conditions where DNA damage accumulates over time. The role of neural stem cell pools and their differentiation capacity is pivotal in sustaining brain function, and persistent DNA lesions could fundamentally compromise this regenerative potential. The study elegantly integrates molecular pathways with cellular phenotypes, contributing to a holistic picture of neural integrity in health and disease.
The paper’s methodological rigor, combined with its translational relevance, sets a benchmark for future studies aiming to dissect the nexus between DNA repair and neurogenesis. Chen and colleagues’ findings invite a reevaluation of therapeutic timelines, emphasizing the need to intervene early in the course of neurodegenerative diseases before irreversible neural network damage ensues. This work serves as a catalyst for multidisciplinary collaborations bridging genetics, stem cell biology, and clinical neurology.
In summary, the elucidation of how novel APTX mutations cause defective neural differentiation through the accumulation of DNA single-strand breaks adds a compelling chapter to the story of neurodegeneration. It highlights the intricate cellular choreography required to maintain DNA integrity during CNS development and the devastating consequences when this balance falters. The study not only advances fundamental science but also sparks optimism for innovative therapies grounded in genomic maintenance.
Future directions inspired by this landmark study include the exploration of combinatorial gene therapies and targeted pharmacological treatments to ameliorate DNA repair defects. Additionally, expanding this research to include in vivo models and patient clinical trials will be paramount in translating these laboratory insights into tangible clinical benefits. The intersection of genome stability and neural development remains a fertile ground for discovery, with this work representing a significant stride towards unraveling the complexities of neurogenetic diseases.
As scientific communities worldwide grapple with neurodegeneration’s growing burden, studies like this underscore the power of integrating stem cell technology with genetic insights to reveal novel pathological paradigms. The convergence of DNA repair defects and neural differentiation failures invites a paradigm shift in how we conceptualize and treat neurological disorders. This research not only charts a course for new therapies but also enriches our fundamental understanding of brain biology.
Subject of Research: The role of novel APTX mutations in neural differentiation defects and accumulation of DNA single-strand breaks using induced pluripotent stem cell models.
Article Title: Induced pluripotent stem cells carrying novel APTX mutations presented defective neural differentiation with the accumulation of DNA single-strand breaks.
Article References:
Chen, Z., Huang, Y., Yuan, Z. et al. Induced pluripotent stem cells carrying novel APTX mutations presented defective neural differentiation with the accumulation of DNA single-strand breaks. Cell Death Discov. 11, 481 (2025). https://doi.org/10.1038/s41420-025-02723-2
Image Credits: AI Generated