In a groundbreaking study published in the prestigious journal Oncotarget on May 19, 2025, researchers from Hamad Bin Khalifa University in Qatar have illuminated a critical biological mechanism that holds promising implications for cancer treatment strategies. The study, titled “PRDX1 protects ATM from arsenite-induced proteotoxicity and maintains its stability during DNA damage signaling,” uncovers the previously unrecognized role of the protein PRDX1 in safeguarding the integrity of the ATM protein—a central orchestrator of DNA repair pathways—particularly under conditions of arsenite-induced cellular stress. This discovery adds a new dimension to our understanding of cellular defense mechanisms and suggests a novel therapeutic target to overcome chemo-resistance in ovarian cancer and potentially other malignancies.
Proteins that maintain genomic stability are essential in cellular defense against DNA damage, a process particularly relevant in the context of cancer, where DNA repair pathways are often dysregulated. ATM (ataxia-telangiectasia mutated) is a serine/threonine kinase activated by DNA double-strand breaks and initiates a cascade of events enabling DNA repair, cell cycle arrest, or apoptosis. The stability and function of ATM are thus pivotal for the cell’s ability to maintain genetic fidelity. Until now, the factors that protect ATM from degradation or functional impairment in the face of oxidative and chemical insults remained elusive.
This new research reveals that PRDX1, widely recognized as an antioxidant enzyme that mitigates oxidative stress by neutralizing reactive oxygen species, has a protective role extending beyond redox homeostasis. Specifically, PRDX1 maintains ATM stability by shielding it from proteotoxic damage induced by arsenite exposure—arsenite being an environmental toxicant well documented to induce proteotoxic stress and DNA damage. In the absence of PRDX1, ATM protein levels rapidly decline when cells face arsenite stress, leading to compromised DNA repair competency. This mechanistic insight elucidates a fundamental vulnerability in the DNA damage response system.
Employing a combination of advanced molecular biology techniques, the investigators demonstrated that PRDX1 physically interacts with ATM, thereby preventing its misfolding and proteotoxic degradation. The loss of PRDX1 disrupted this protective interaction, leaving ATM susceptible to arsenite-induced ubiquitination and subsequent proteasomal degradation. This degradation cascade effectively debilitated downstream DNA damage signaling and repair pathways, underscoring the indispensable role of PRDX1 as a guardian of genomic integrity.
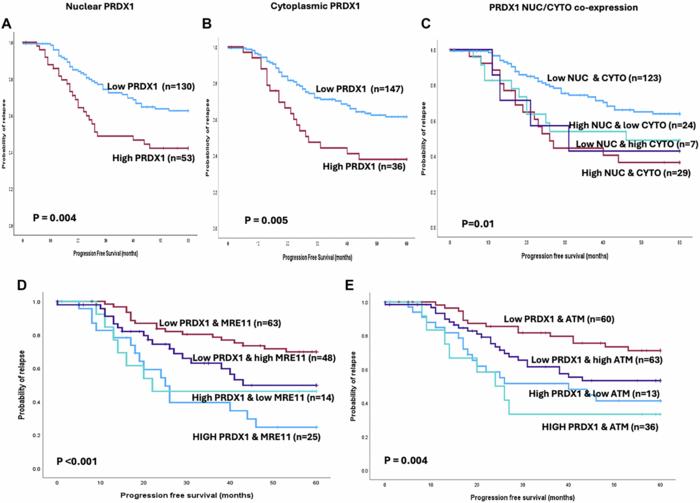
The translational relevance of these findings was underscored by analyses of clinical ovarian cancer samples. High tumor expression levels of PRDX1 consistently correlated with elevated ATM and MRE11 protein levels—MRE11 being a critical nuclease in homologous recombination repair. This co-expression profiles aligned with more aggressive tumor phenotypes and poorer patient progression-free survival metrics, suggesting that such tumors could be leveraging the PRDX1-ATM axis to fortify their DNA repair machinery and evade the cytotoxicity of platinum-based chemotherapies.
Intriguingly, experimental inhibition or genetic ablation of PRDX1 sensitized cancer cells to chemotherapy, notably platinum drugs that inflict DNA crosslinks and strand breaks. When combined with low doses of arsenite, which on its own induces proteotoxic stress, the absence of PRDX1 amplified DNA damage-induced cytotoxicity. Moreover, co-treatment with ATM inhibitors synergized with arsenite exposure to further compromise cancer cell viability. This multimodal assault suggests a new therapeutic paradigm focused on disabling the PRDX1 shield to unleash the full efficacy of DNA-damaging agents.
Cancer cells notorious for their intrinsic or acquired chemoresistance often possess augmented DNA repair capabilities that facilitate the survival of DNA lesions inflicted by treatment. Therefore, targeting PRDX1 offers an innovative avenue to undermine this defense, rendering tumor cells more vulnerable to conventional and targeted therapies. The potential of small molecule PRDX1 inhibitors or leveraging genetic variants in PRDX1 that impair its function could be exploited to design combinatorial treatments tailored to resistant cancer phenotypes.
Beyond ovarian cancer, the implications of these mechanistic insights extend broadly across oncology given the universal reliance of proliferating cells on ATM-mediated DNA repair. The interplay between redox regulation and DNA repair stability, as highlighted by the PRDX1-ATM interaction, uncovers a node of cellular vulnerability that may be exploited across multiple tumor types. Moreover, the study highlights the dualistic role of PRDX1, emphasizing its protective capacity in normal cells but deleterious potential in cancer cells by bolstering their defense against therapeutic DNA damage.
This discovery further positions PRDX1 as not only a therapeutic target but also a biomarker with prognostic value in predicting patient response to DNA damage-based chemotherapies. Stratifying patients based on tumor PRDX1 expression and functional status could inform precision medicine approaches, optimizing treatment regimens and improving clinical outcomes by identifying who may benefit from PRDX1-targeted interventions or arsenite-sensitized therapy.
Methodologically, the study employed state-of-the-art biochemical assays, survival analyses with large ovarian cancer patient cohorts, and rigorous molecular genetic approaches to dissect this intricate protein interplay. The Kaplan-Meier survival curves illustrated the clinical repercussions of PRDX1 expression and its synergy with ATM and MRE11, cementing a clinically actionable relationship between DNA repair capacity and patient survival. This robust integration of molecular biology and clinical data strengthens the translational impact of the findings.
Furthermore, the study contributes significantly to the broader understanding of arsenic toxicity mechanisms, a global public health concern due to arsenic contamination in drinking water and environmental exposure. By delineating how arsenite induces proteotoxic stress that destabilizes essential DNA repair proteins, this research adds critical knowledge to toxicogenomics and cellular stress response paradigms, opening avenues for mitigating arsenic-related carcinogenesis.
In conclusion, the work from Reem Ali, Dindial Ramotar, and colleagues not only expands the functional repertoire of PRDX1 but also advocates for novel therapeutic strategies that combine PRDX1 inhibition with low-dose arsenite and DNA repair inhibitors. This approach promises to transform the management of chemoresistant tumors by exploiting inherent dependencies in their DNA repair machinery. As the oncology field advances toward precision and combinatorial therapies, this study underscores the importance of fundamental molecular insights in catalyzing clinical innovations.
Subject of Research: Cells
Article Title: PRDX1 protects ATM from arsenite-induced proteotoxicity and maintains its stability during DNA damage signaling
News Publication Date: 19-May-2025
Web References:
- Oncotarget Volume 16: https://www.oncotarget.com/archive/v16/
- DOI: http://dx.doi.org/10.18632/oncotarget.28720
Image Credits:
Copyright © 2025 Ali et al. This is an open access article distributed under the Creative Commons Attribution License (CC BY 4.0), permitting unrestricted use, distribution, and reproduction.
Keywords:
cancer, redox signaling, homologous recombination, protein interaction, cell cycle, protein modification