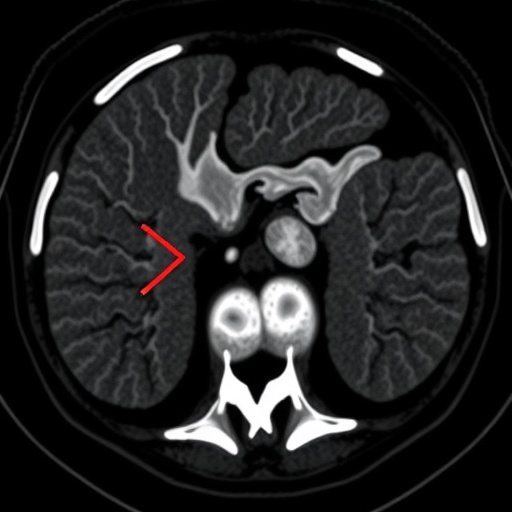
In a groundbreaking study recently published in BMC Cancer, researchers have unveiled detailed imaging characteristics of eosinophilic solid and cystic renal cell carcinoma (ESC-RCC), a rare subtype of renal malignancy. This comprehensive analysis, which encompassed imaging data from seven patients over a four-year period, offers novel insights into the diagnostic nuances that differentiate ESC-RCC from other renal tumors, potentially reshaping approaches in radiological assessment and clinical decision-making.
ESC-RCC presents a complex diagnostic challenge due to its rarity and overlapping features with other renal neoplasms. The study’s retrospective review focused on seven patients, balanced between genders with a median age of 46, highlighting the tumor’s occurrence across a diverse age spectrum. Interestingly, while the majority of cases involved unilateral solitary lesions, one patient exhibited bilateral multifocal involvement, emphasizing the heterogeneous clinical manifestations of this cancer subtype.
Tumor size varied considerably, ranging from 2.0 to 8.5 centimeters, with an average diameter of approximately 4 centimeters. This variability underscores the importance of meticulous imaging evaluation in identifying lesions at an early stage. On non-contrast computed tomography (CT), most tumors demonstrated a mixed density pattern, with alternating high and low areas suggestive of their eosinophilic and cystic components. This heterogeneous appearance is vital for radiologists to recognize, as it contrasts sharply with the uniform densities seen in other renal masses.
Contrast-enhanced CT scanning revealed even more nuanced information. About 75% of tumors exhibited heterogeneous enhancement, a finding that further delineates the unique vascular architecture of ESC-RCC. Enhancement peaks occurred predominantly during the corticomedullary and nephrographic phases, highlighting dynamic vascular changes as contrast material perfuses the tumor. These imaging phases correspond to early and late post-contrast intervals, respectively, which are critical windows for capturing oncological vascular patterns.
Significantly, all tumors demonstrated distinct boundaries with clear demarcation from surrounding renal parenchyma. This well-defined nature, coupled with the absence of calcifications, necrosis, or hemorrhage, aids clinicians in differentiating ESC-RCC from more aggressive renal cancers known for invasive growth and internal bleeding. Moreover, the lack of lymph node metastasis observed microscopically across all cases offers a hopeful prognosis for patients diagnosed with this subtype.
The study also noted vascular compression in three-quarters of the tumors, indicating that although ESC-RCCs are well-circumscribed, they can exert pressure on adjacent vascular structures. The presence of floating vessels—seen in nearly 88% of cases—within or adjacent to the tumor mass may reflect the tumor’s angiogenic behavior and could serve as an imaging hallmark in clinical practice, guiding surgical planning and risk stratification.
Tumor classification in this cohort identified a predominance of type III lesions, with smaller proportions of types II and IV, and only a single case categorized as type I, providing a framework for future research to explore the biological implications of these subtypes. Such classification schemes, when coupled with imaging data, could facilitate personalized therapeutic strategies and targeted treatment modalities.
From a technical perspective, the study’s methodology underscores the value of integrating multimodal imaging techniques to unravel the complex nature of ESC-RCC. High-resolution CT scans, combined with contrast enhancement phases, allowed for detailed visualization of tumor heterogeneity, vascular relationships, and enhancement kinetics—parameters essential for establishing diagnostic criteria in clinical oncology and radiology.
The absence of calcifications, fat, and hemorrhagic components in the tumors is particularly noteworthy. These characteristics sharply contrast with features commonly observed in other renal tumors, including clear cell and papillary renal cell carcinomas, which often display calcified foci or intratumoral bleeding. Recognizing these distinctive imaging traits can substantially improve diagnostic confidence and reduce the need for invasive biopsies.
Clinically, this study’s findings reinforce the importance of considering ESC-RCC in the differential diagnosis when encountering well-demarcated cystic-solid renal masses. The unique enhancement pattern—where the degree of tumor enhancement remains consistently lower than that of the adjacent normal renal cortex across all contrast phases—emerges as a signature feature, potentially serving as a non-invasive imaging biomarker for ESC-RCC.
Furthermore, the study advocates for heightened awareness among radiologists and urologists regarding this rare tumor type. Early and accurate detection through imaging not only facilitates timely surgical intervention but also provides prognostic insights, given the tumor’s lack of aggressive pathological features such as necrosis and lymphatic spread.
Future research directions prompted by this work include the exploration of advanced imaging modalities such as multiparametric MRI and molecular imaging techniques to further refine diagnostic accuracy. Investigations into the tumor’s genetic and molecular landscape, correlated with imaging phenotypes, may unlock new avenues for targeted therapies and personalized medicine approaches.
In summary, the detailed imaging characterization of eosinophilic solid and cystic renal cell carcinoma by Ling et al. represents a significant advancement in renal oncology. By delineating the tumor’s unique radiographic profile—marked by well-demarcated cystic-solid morphology, distinctive enhancement patterns, and the absence of calcification or hemorrhage—this study lays the groundwork for improved diagnosis and management of this enigmatic renal neoplasm.
As awareness of ESC-RCC grows within the medical community, it is anticipated that such research will catalyze the development of more precise diagnostic algorithms and treatment guidelines. The integration of radiological expertise with pathological validation remains paramount in conquering the challenges posed by rare tumor entities like ESC-RCC.
With the continued evolution of imaging technologies and deeper understanding of tumor biology, clinicians are better equipped than ever to detect and characterize complex renal masses. This study not only enriches the current literature but also emboldens radiologists to incorporate these imaging hallmarks into routine practice, ultimately enhancing patient outcomes through early and accurate diagnosis.
Subject of Research: Imaging characteristics and differential diagnosis of eosinophilic solid and cystic renal cell carcinoma.
Article Title: Imaging features of eosinophilic solid and cystic renal cell carcinoma
Article References:
Ling, C., Li, J., Tan, R. et al. Imaging features of eosinophilic solid and cystic renal cell carcinoma. BMC Cancer 25, 1596 (2025). https://doi.org/10.1186/s12885-025-15065-0
Image Credits: Scienmag.com