A paradigm shift is underway in the realm of organic chemistry, as researchers at Hokkaido University delve into the uncharted territories of mechanochemical synthesis. In a groundbreaking study, they reveal how macroscopic mechanical forces can significantly influence reaction rates within a ball mill, offering new insights that challenge traditional perceptions of organic synthesis. This research not only augments the mechanochemical methodology but underscores its eco-friendly advantages over conventional approaches, which typically rely on solvents that generate industrial waste.
Mechanochemistry, characterized by its solvent-free environment, stands out for its potential to utilize solid-state reactants, which often don’t dissolve adequately in standard solvents. This innovation aligns seamlessly with modern sustainability goals, attracting considerable interest within the scientific community. Yet, despite the promising implications of mechanochemistry, a profound understanding of its underlying kinetics remains scant. Conventional organic synthesis methods have been well-established for decades, so the theoretical framework surrounding mechanochemical processes is still developing, which is a vital step toward its acceptance as a mainstream strategy.
Led by Associate Professor Tetsuya Yamamoto from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), the research team has taken significant strides in bridging this gap. Their findings, published in the esteemed journal RSC Mechanochemistry, articulate a new theory predicting chemical reaction kinetics in mechanochemical contexts, particularly focused on the interaction dynamics within a ball mill. This collaborative effort—melding expertise from both organic chemistry and rheology—illustrates the interdisciplinary nature of modern scientific inquiry.
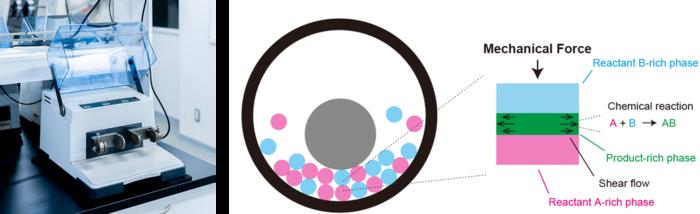
The heart of this theory lies in the interface between solid reactants, where the chemistry unfolds. When reactants in a ball mill collide, mechanical forces are exerted not only on the bulk materials but significantly at their contact points. This leads to the formation of a thin layer of products at the interface, which the newly developed theory posits as a critical factor governing reaction rates. As the balls collide, they compress the product-rich layer, reducing its thickness and facilitating more rapid and effective collisions between the remaining reactants.
This phenomenon offers a compelling explanation for the acceleration of mechanochemical reactions. The theory suggests that the enhancement in reaction speed is fundamentally linked to the efficiency of these interactions, wherein the application of force mitigates obstacles at the molecular level. It is this realization that paves the way for a deeper and more nuanced understanding of how mechanochemical reactions can be optimized.
Associate Professor Tetsuya Yamamoto, the illustrious first author of the study, heralds this work as a critical first step toward developing a kinetic theory specifically tailored for mechanochemical reactions centered around interfacial phenomena. His enthusiasm reflects the thrill of pioneering research, as he notes the importance of collaborative efforts in achieving scientific breakthroughs. The complexities of these mechanochemical processes have historically eluded thorough elucidation through experimental means alone, underscoring the necessity of theoretical frameworks to anchor future explorations.
The collaborative synergy within the WPI-ICReDD has been central to advancing this field, with Associate Professor Koji Kubota, the study’s second author, emphasizing the strategic blend of disciplines. This research signifies a significant departure from past methodologies that confined mechanochemistry to empirical observation, thereby enabling deeper insights into the mechanistic pathways that underpin it. Understanding these pathways is imperative as it lays the groundwork for future improvements in reaction design and efficacy.
The implications of this theoretical advancement are substantial, not only for academic inquiry but also for practical applications in industry. By refining our grasp of mechanochemical synthesis, it becomes possible to enhance product yields and reaction efficiencies, opening doors to novel organic compound production processes. The environmental benefits of reducing solvent use echo the global shift toward greener chemistry, aligning research advancements with societal expectations for sustainability.
Furthermore, this article contributes to a broader narrative of mechanochemistry, revealing it as a versatile alternative that holds promise for various industries, including pharmaceuticals and materials science. As research continues to unfold, the balance between theoretical insights and experimental validation will be pivotal in confirming the robustness of this new framework and its predictive capabilities.
This research collective’s journey epitomizes the quintessential spirit of innovation in science, where overcoming established paradigms leads to newfound understanding. As the scientific community continues to grapple with the complexities of mechanochemical processes, the insights derived from this work will undoubtedly inspire a new generation of research that further explores the intersection of force, chemistry, and sustainability.
In conclusion, the emerging theoretical framework offered by Yamamoto and his team represents a significant leap toward comprehending the kinetics of mechanochemical reactions. As a newfound lens through which scientists can assess these processes, it sets the stage for further advancements that promise to revolutionize organic synthesis methodologies in the coming years.
Subject of Research: Mechanochemical organic synthesis
Article Title: Scaling theory for the kinetics of mechanochemical reactions with convective flow
News Publication Date: December 7, 2024
Web References: 10.1039/D4MR00091A
References: N/A
Image Credits: Photo