A groundbreaking advancement in the fight against glioblastoma, one of the deadliest and most treatment-resistant brain tumors, has emerged from researchers at the San Raffaele-Telethon Institute for Gene Therapy (SR-TIGET) in Milan. This multidisciplinary team, led by Nadia Coltella and Luigi Naldini, has devised an innovative gene therapy strategy designed to restore and enhance the antitumor efficacy of chimeric antigen receptor (CAR) T cell therapy within the hostile environment of solid tumors. Published in Science Translational Medicine, their study reveals how precise cytokine delivery into the tumor microenvironment (TME) can effectively reprogram immune cells and reinvigorate CAR T cell activity, thus altering the course of tumor progression in comprehensive preclinical glioblastoma models.
Glioblastoma’s resistance to current therapies has long been attributed in large part to its immunosuppressive microenvironment, which severely limits the infiltration, survival, and function of therapeutic T cells. Traditional CAR T cell therapies that have demonstrated remarkable success against hematological malignancies face formidable obstacles when targeting solid tumors like glioblastoma. The lack of sustained T cell activation and the prevalence of exhaustion phenotypes contribute to their diminished efficacy. Addressing this formidable challenge, the research team exploited a gene therapy approach that genetically engineers hematopoietic progenitor cells to produce monocytes and macrophages capable of selectively releasing immunostimulatory cytokines upon tumor infiltration.
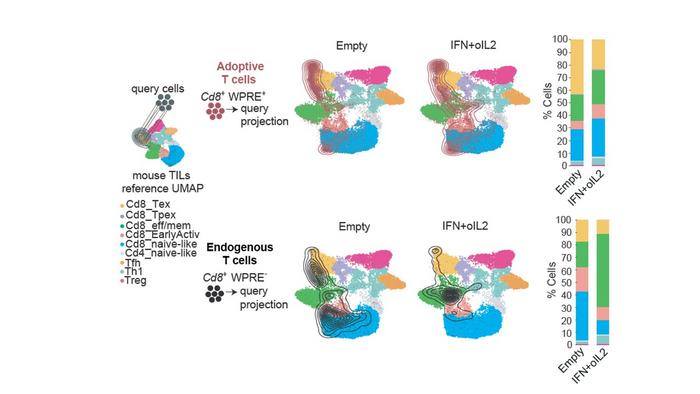
This precision delivery system utilizes a dual-cytokine strategy within the TME. Interferon-alpha (IFN-α), known for its multifaceted immune-enhancing properties, counters immunosuppressive cues, improves antigen presentation, and amplifies the activity of immune effector cells. Concurrently, an engineered mutation of interleukin-2 (IL-2) selectively activates a mutated receptor expressed exclusively on the administered CAR T cells, ensuring that only these therapeutic cells receive proliferative signals without triggering systemic toxicities. The co-expression of this mutant IL-2 receptor and the mutant cytokine creates a private, tumor-confined signaling axis that fine-tunes the immune response.
The study employed murine models that faithfully recapitulate human glioblastoma pathophysiology and immunological barriers, enabling comprehensive evaluation of the therapeutic paradigm. In these models, CAR T cells administered alone demonstrated minimal antitumor effects, mirroring clinical trial outcomes where CAR T cells have struggled to control solid tumors. However, when combined with tumor-targeted cytokine delivery, the CAR T cells exhibited restored functional potency, resulting in pronounced delays in tumor growth and significant improvements in survival. Notably, the antitumor response extended even to tumors with heterogeneous antigen expression, suggesting that the approach transcends single antigen targeting by enlisting endogenous T cells through a mechanism known as antigenic spreading.
Antigenic spreading is a critical immune phenomenon whereby an initial immune response against a specific tumor antigen broadens to include additional tumor-associated antigens. This results in a more robust and comprehensive antitumor immunity capable of counteracting tumor immune evasion strategies. The researchers found that IFN-α played a pivotal role in orchestrating this process by reshaping the TME into an immune-stimulatory milieu that recruits and activates the host’s own T cell populations alongside the engineered CAR T cells. This cooperative engagement of multiple immune cell subsets provides a promising foundation for durable tumor control.
Central to the approach is the reprogramming of tumor-associated macrophages, which are ordinarily contributors to the immunosuppressive environment. By incorporating genes encoding the cytokines under tight regulatory control into hematopoietic progenitors, the resulting macrophages deliver the immunostimulatory payload directly within the tumor niche. This localized cytokine release modifies the TME and facilitates a conducive environment for CAR T cell persistence and activation. The spatial precision of this cytokine delivery minimizes systemic exposure, thereby reducing the risk of adverse effects that have hampered previous systemic cytokine therapies.
The concept of private cytokine cross-talk established in this study represents a paradigm shift in immunotherapy. Rather than systemic administration of immune stimulants—which often result in dose-limiting toxicities and off-target effects—this method confines cytokine activity to the precise cellular players and anatomical location implicated in antitumor immunity. According to co-first author Dr. Alvisi, this targeted interaction “ensures that immune stimulants act only where needed, sparing the rest of the body from systemic toxicity, and specifically on the relevant target cells involved in tumor attack.”
This research also builds on the prior success of the gene therapy platform now implemented in a first-in-human clinical trial, the Temferon trial (NCT03866109), conducted by Genenta Science, a biotech spin-off originating from the San Raffaele Institute. Temferon leverages the selective delivery of IFN-α to glioblastoma lesions as a stand-alone treatment, demonstrating feasibility, safety, and preliminary biological activity in human subjects. While early results highlight promising modulation of the tumor microenvironment and hints of therapeutic benefit, the intrinsic limitations of a phase 1 study with a small patient cohort warrant further exploration into combination strategies.
The present study’s demonstration that coupling the Temferon approach with CAR T cell therapy potentiates antitumor efficacy opens exciting avenues for clinical translation. By broadening the therapeutic arsenal against glioblastoma, this combinatory strategy could overcome the historical resistance encountered by cellular immunotherapies targeting solid tumors. The robust engagement of endogenous T cells and the generation of a more permissive microenvironment underscore the potential to combat tumor heterogeneity and immune escape alike.
The implications of this work extend beyond glioblastoma, providing a blueprint for integrating gene therapy-driven macrophage reprogramming with engineered T cell therapies across diverse solid tumor indications. It exemplifies a sophisticated interplay between cellular therapies and localized gene delivery systems to coax the immune system into mounting a potent and selective antitumor response. Such convergence of technologies might redefine therapeutic paradigms in oncology, pushing the boundaries of what is achievable with precision immunotherapy.
Luigi Naldini, Director of SR-TIGET, remarks, “This work represents another important step forward in our decade-long commitment to develop novel gene and cell therapy strategies effective against tumors… A combination of Temferon with CAR T cell administration, as prompted by our new study, could in future further enhance the benefit of the treatment and broaden its efficacy.” This sentiment captures the transformative potential of bridging innovative genetic engineering with advanced cellular therapies to tackle one of the most challenging malignancies known to medicine.
In conclusion, the study presented by the SR-TIGET team delivers a compelling demonstration of how tumor-targeted cytokine delivery can rescue CAR T cell function and orchestrate a coordinated antitumor immune response in glioblastoma. By constructing a private cytokine communication channel within the tumor, the therapy not only revitalizes CAR T cells but also mobilizes broad host immunity, marking a significant stride toward effective immunotherapeutics in solid cancers. The journey from genetic engineering of progenitors to clinical translation exemplifies the power of integrative science to innovate in the fight against cancer, inspiring hope for patients and clinicians alike facing the daunting prognosis of glioblastoma.
Subject of Research: Immunotherapy, Gene Therapy, Glioblastoma, CAR T Cells, Tumor Microenvironment, Cytokine Delivery
Article Title: A cross-talk established by tumor-targeted cytokines rescues CAR T cell activity and engages host T cells against glioblastoma in mice
News Publication Date: 2-Jul-2025
Web References:
- DOI: 10.1126/scitranslmed.ado9511
- Temferon Trial: NCT03866109
- San Raffaele-Telethon Institute for Gene Therapy (SR-TIGET): https://research.hsr.it/en/institutes/san-raffaele-telethon-institute-for-gene-therapy.html
- Genenta Science: https://www.genenta.com/
Image Credits: San Raffaele-Telethon Institute for Gene Therapy (SR-TIGET)
Keywords: Glioblastoma, CAR T Cells, Gene Therapy, Cytokines, Tumor Microenvironment, IFN-α, Interleukin-2, Macrophage Reprogramming, Immune Cross-talk, Antigenic Spreading, Temferon, Solid Tumor Immunotherapy