In an unprecedented synthesis of decades-long research, a groundbreaking review published in the journal Brain Medicine profoundly reshapes our understanding of electroconvulsive therapy (ECT) dosing for depression. Despite its controversial history, ECT remains one of the most efficacious treatments for severe depressive disorders. This comprehensive analysis delves into the nuanced relationship between the number of ECT sessions and therapeutic outcomes, illuminating the delicate balance between clinical efficacy and cognitive side effects.
Traditionally, the psychiatric community has adhered to relatively rigid treatment regimens, typically advocating between six to twelve ECT sessions. However, the new review spearheaded by Professor Yanghua Tian from Anhui Medical University challenges this convention by revealing a more complex dose-response curve. Their findings indicate that the bulk of symptomatic relief occurs early in the treatment course, with approximately a 26% reduction in depression severity following just one session, and nearly 50% improvement by the third. This rapid early response suggests that the conventional extended course model may not be universally necessary or optimal.
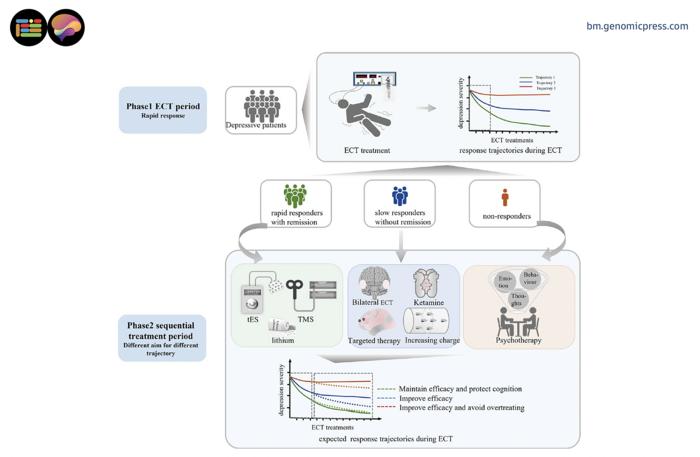
A central revelation from this review is the identification of distinct patient response trajectories. Approximately one-quarter of patients exhibit swift remission within a handful of sessions, while a significant subset shows moderate, incremental improvements. Alarmingly, a notable minority—roughly 13%—display minimal response even after prolonged ECT courses. This heterogeneity suggests the potential of tailored treatment strategies, rather than the prevailing one-size-fits-all protocols. It raises the pressing clinical question of how to preemptively discern responders from non-responders or slow responders.
Such distinctions are not academic but critical, because prolonged ECT administration carries a quantifiable cognitive toll. Memory impairment and other cognitive deficits emerge surprisingly early, sometimes as soon as the second ECT session, accumulating with subsequent treatments. This cumulative burden presents a clinical paradox: while additional sessions may provide limited incremental benefit for some, they simultaneously compound patients’ cognitive risks. This finding underscores the need for precision dosing and stringent monitoring throughout the treatment course.
The paradigm shift proposed by the authors advocates for a "response-guided sequential treatment" approach. Instead of adhering to a fixed number of sessions prescribed a priori, this strategy calls for dynamic adjustments based on real-time patient response. Notably, ECT is envisioned as the initial ‘leg’ of a relay, delivering rapid mood stabilization within 3 to 6 sessions for responders, after which care transitions toward safer maintenance therapies such as non-invasive brain stimulation techniques or psychotherapy. For slow or non-responders, alternatives like ketamine augmentation or entirely different modalities might be indicated earlier in the treatment course.
Underlying this shift are compelling neurobiological insights that unveil how the brain adapts during ECT. Advanced neuroimaging reveals that hippocampal volume—central to memory processing—progressively increases through the treatment course. This volumetric plasticity aligns with changes in neurotransmitter systems and inflammatory markers, highlighting a complex adaptive cascade triggered by induced seizures. These dynamic processes imply that the brain does more than merely endure ECT; it actively remodels itself, with implications for both therapeutic efficacy and adverse side effects.
Interestingly, seizure duration has traditionally been employed as a marker of treatment adequacy within ECT protocols, with longer seizures often equated to better outcomes. However, the review documents a marked decrease in seizure duration over successive sessions. This counterintuitive trend provokes pivotal questions: does declining seizure length indicate emerging treatment resistance, or is it reflective of adaptive neural mechanisms mitigating seizure propagation? Addressing this could lead to refinements in how treatment adequacy is assessed in clinical practice.
The integration of biomarkers, including serum brain-derived neurotrophic factor (BDNF) and cortisol levels, adds a further dimension to understanding ECT’s neurophysiological footprint. These biomarkers exhibit transient spikes immediately post-session, normalizing over time, painting a picture of highly dynamic systemic responses. Such patterns evoke the tantalizing possibility that future treatment could be guided not solely by clinical scales but augmented by objective biological signals, enhancing personalized treatment strategies.
The implications for psychiatry are potentially transformative. By coupling rigorous symptom monitoring with cognitive assessments and biomarker data, clinicians might soon implement more agile, patient-specific regimens that maximize remission while sparing cognitive function. This approach could challenge entrenched treatment paradigms and reduce the stigma often associated with ECT’s cognitive side effects, thereby improving patient acceptance and access to this life-saving intervention.
Beyond individual patient benefits, the potential system-level advantages are notable. Given that many healthcare systems face limited ECT capacity and increasing depression prevalence, adopting a response-guided methodology could optimize resource utilization. Shorter average treatment parcels without compromising efficacy would enable more patients to benefit from ECT. Equally, patients hesitant to undergo ECT owing to fears of cognitive decline may find reassurance in protocols designed to minimize exposure.
However, numerous questions remain unanswered, inviting further rigorous research. The long-term relapse rates following early termination of ECT and transition to adjunctive therapies warrant careful investigation. Whether combination approaches initiated after fewer ECT sessions may outperform extended monotherapy remains an open query. Additionally, the integration of emerging neurostimulation technologies like transcranial magnetic stimulation within these sequential frameworks poses exciting possibilities for future clinical innovation.
The review’s authors advocate for the development of predictive algorithms integrating clinical features, imaging, biomarkers, and early response patterns to refine individualized treatment plans. Such precision medicine approaches are well poised to revolutionize psychiatric practice, ushering in an era where ECT is wielded with surgical precision, maximizing benefit while mitigating harm.
In summation, this landmark review elucidates a new era in ECT treatment, moving away from rigid, protocol-driven courses to adaptive, response-informed strategies. It heralds a future in which ECT’s profound efficacy in combating severe depression is harnessed with unprecedented accuracy and safety. As mental health challenges continue to mount globally, such innovations promise to reshape therapeutic paradigms, offering hope for improved outcomes and enhanced quality of life for millions living with treatment-resistant depression.
Subject of Research: People
Article Title: Rethinking the impact and management of electroconvulsive therapy session number in depression
News Publication Date: 3 June 2025
References:
Tian, Y., Ji, Y., et al. (2025). Rethinking the impact and management of electroconvulsive therapy session number in depression. Brain Medicine. DOI: 10.61373/bm025i.0053
Image Credits: Yanghua Tian
Keywords: Electroconvulsive therapy, ECT dosing, depression treatment, cognitive side effects, response-guided therapy, neurobiology, biomarkers, hippocampal plasticity, precision psychiatry, treatment-resistant depression