Ovarian cancer remains one of the deadliest gynecological malignancies worldwide, with an alarmingly low five-year survival rate for patients diagnosed at advanced stages. Central to its fatal progression is the peritoneal metastasis, a complex biological phenomenon that enables cancer cells to disseminate within the abdominal cavity. Despite extensive research, therapeutic interventions have seen limited success, largely due to the elusive nature of the underlying molecular drivers orchestrating metastatic dissemination. Recent scientific endeavors have shifted focus to the epithelial-mesenchymal transition (EMT), a dynamic cellular program that endows epithelial cells with mesenchymal properties, enhancing their motility and invasiveness. Yet, direct pharmacological targeting of EMT remains an unsolved challenge because traditional transcriptional regulators involved in this transition often play vital roles in normal tissue maintenance, and exhibit redundancy.
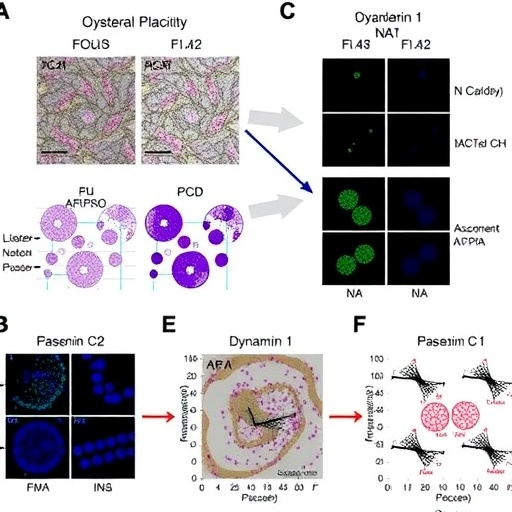
Emerging from this landscape, a groundbreaking study has brought to light a novel non-transcriptional regulator intricately involved in sustaining the plastic mesenchymal state characteristic of metastatic ovarian cancer cells. At the heart of this discovery is Dynamin 1 (DNM1), a GTPase classically known for its pivotal role in endocytosis. Using integrative bioinformatics analyses across The Cancer Genome Atlas (TCGA) datasets, encompassing a broad spectrum of over 8,000 patient samples from 20 cancer types, researchers employed advanced computational frameworks including master regulator algorithms and the Algorithm for the Reconstruction of Accurate Cellular Networks (ARACNE). This comprehensive approach unveiled markedly elevated expression of DNM1 in ovarian cancer patients exhibiting advanced clinical stages and mesenchymal subtypes, with a compelling correlation to diminished progression-free and post-relapse survival, positioning DNM1 as a putative driver of tumor aggressiveness.
Functionally dissecting DNM1’s contribution, in vitro experiments delineated its crucial role in regulating EMT phenotypes. Silencing DNM1 expression in highly metastatic ovarian cancer cell lines resulted in a significant attenuation of mesenchymal traits—cells exhibited reduced migratory capacity and decreased expression of N-cadherin, a key adhesion molecule intimately linked to EMT and metastatic competence. Conversely, ectopic overexpression of DNM1 in otherwise non-metastatic ovarian cancer cells induced a pronounced acquisition of invasive characteristics, concomitant with upregulated N-cadherin. These functional modulations underscore the indispensable role of DNM1 in tuning the dynamic equilibrium between epithelial and mesenchymal states.
Validating these findings in vivo, murine models of peritoneal metastasis reinforced the functional indispensability of DNM1 in metastatic colonization. Mice bearing ovarian tumors with targeted DNM1 knockdown manifested a stark reduction in tumor dissemination across the peritoneum, corroborating the in vitro insights and implicating DNM1 as a formidable mediator of metastatic colonization. Delving deeper into mechanistic pathways, the research revealed that DNM1 orchestrates EMT progression by regulating the endocytic recycling of glycosylated N-cadherin. This post-translational trafficking mechanism sustains the plasticity and polarity of mesenchymal cancer cells, facilitating enhanced migratory and invasive capacities essential for metastasis.
Complementing these mechanistic insights, integrated epigenomic and transcriptomic analyses, harnessing ATAC-seq and RNA-seq technologies, unveiled a potential suppressive axis in non-metastatic ovarian cancer cells. The enzyme beta-1,3-galactosyltransferase 1 (B3GALT1) emerged as upregulated in such contexts, hypothesized to antagonize EMT by impeding the recycling of N-cadherin, thereby dampening mesenchymal plasticity. This finding not only delineates differential regulatory landscapes underpinning metastatic versus non-metastatic states but also suggests a complex interplay between glycosylation enzymes and endocytic trafficking in cancer progression.
Beyond molecular intricacies, the study illuminated surprising therapeutic implications relating to nanoparticle-mediated drug delivery. Metastatic ovarian cancer cells characterized by heightened DNM1 activity displayed increased sensitivity to nanoparticle uptake, attributed to their augmented endocytic machinery. This phenomenon hints at the tantalizing prospect of exploiting DNM1-mediated endocytosis to enhance the efficacy of nanodrugs, potentially ushering in a paradigm shift in targeted ovarian cancer therapies that transcends conventional approaches.
Taken together, these findings establish a previously unrecognized DNM1-N-cadherin axis as a fundamental regulator of EMT plasticity and metastatic virulence in ovarian cancer. By linking endocytic trafficking mechanisms to cellular phenotype regulation, the research bridges pivotal gaps in our understanding of metastatic biology. The identification of DNM1 as both a biomarker and mechanistic driver opens promising therapeutic avenues, including targeted nanodrug delivery strategies that capitalize on inherent cellular vulnerabilities.
This paradigm-shifting work, titled “Dynamin 1-mediated endocytic recycling of glycosylated N-cadherin sustains the plastic mesenchymal state to promote ovarian cancer metastasis”, was published on April 9, 2025 in the journal Protein & Cell. It exemplifies how integrating multi-omic datasets with experimental rigor can unravel complex cancer biology, moving beyond traditional transcription factor-centric models toward novel non-transcriptional pathways with translational relevance.
Beyond expanding our molecular grasp of ovarian cancer metastasis, these insights may transcend this malignancy, as DNM1 and endocytic pathways are likely implicated in diverse tumor contexts exhibiting EMT-driven dissemination. Future research should prioritize the development of specific inhibitors targeting DNM1-mediated endocytic recycling and refine nanotherapeutic carriers tailored to exploit this pathway. Such innovations promise to significantly advance precision oncology, offering hope to patients grappling with aggressive and metastatic ovarian cancer.
Ultimately, this pioneering study underscores the transformative potential of targeting non-transcriptional regulators of EMT, particularly those involved in membrane trafficking and protein recycling, a frontier that until now has remained largely unexplored. By charting this novel territory, the research champions a new era of metastasis-targeted therapies with the potential to improve clinical outcomes for one of the most challenging cancers faced by modern medicine.
Article Title: Dynamin 1-mediated endocytic recycling of glycosylated N-cadherin sustains the plastic mesenchymal state to promote ovarian cancer metastasis
News Publication Date: 9-Apr-2025
Web References: https://doi.org/10.1093/procel/pwaf019
Image Credits: Yuee Cai, Zhangyan Guan, Yin Tong, Weiyang Zhao, Jiangwen Zhang, Ling Peng, Philip P. C. Ip, Sally K. Y. To, Alice S. T. Wong
Keywords: Ovarian cancer, Epithelial-mesenchymal transition, Dynamin 1, Endocytic recycling, N-cadherin, Metastasis, Glycosylation, Nanoparticle uptake, Biomarker, Targeted therapy