In a groundbreaking study that bridges nutrition, immunology, and oncology, researchers have unveiled compelling evidence that dietary restriction can fundamentally reprogram the fate of CD8+ T cells, thereby enhancing anti-tumor immunity and bolstering responses to immunotherapy. This discovery, published in Nature Metabolism, promises to reshape current paradigms surrounding cancer treatment and immune system modulation, offering a potent, non-pharmaceutical avenue to amplify the efficacy of cutting-edge therapies targeting malignant tumors.
At the heart of this research lies the enigmatic relationship between metabolism and immune cell function. CD8+ T cells, essential players in the immune system’s cytotoxic arsenal, are responsible for identifying and destroying cancer cells. However, their efficacy is often compromised within the immunosuppressive microenvironment of tumors. The investigators posited that altering systemic metabolic conditions through dietary restriction could recalibrate the metabolic programming of these T cells, enhancing their cytotoxic potential against tumors.
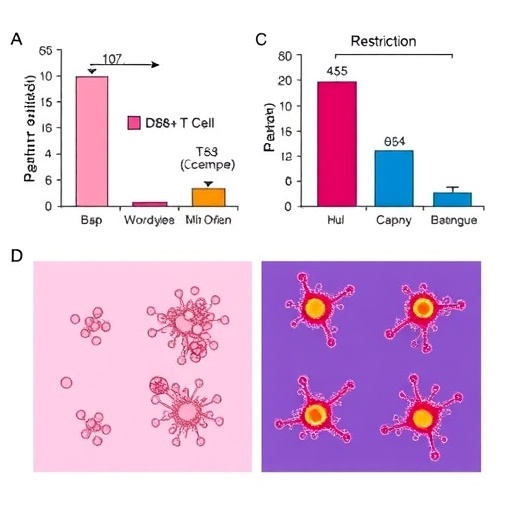
The study utilized sophisticated mouse models of cancer combined with rigorous immunological assays to dissect the influence of dietary restriction regimes. These regimes ranged from caloric reduction to specific nutrient limitations, aiming to mimic clinically feasible dietary interventions. Intriguingly, they observed that such dietary restrictions stimulated a metabolic shift in CD8+ T cells, favoring oxidative phosphorylation over glycolysis, a hallmark of long-lived memory T cells known for their robust and sustained anti-tumor responses.
This metabolic reprogramming was accompanied by profound functional changes in CD8+ T cells. Post dietary intervention, these cells exhibited elevated expression of transcription factors associated with memory differentiation, enhanced mitochondrial biogenesis, and increased production of cytotoxic molecules such as perforin and granzyme B. These molecular hallmarks translate into an augmented capacity for tumor cell killing, which was confirmed by reduced tumor growth rates in treated animals.
Beyond intrinsic changes in T cell metabolism, the researchers discovered that dietary restriction modulated systemic factors such as reduced circulating levels of insulin-like growth factor 1 (IGF-1) and altered amino acid availability, both of which are known to influence T cell fate decisions. The lowered IGF-1 signaling pathway activity appears to remove inhibitory constraints on T cell differentiation, tipping the balance toward memory cell development and longevity, essential parameters for durable anti-cancer immunity.
Crucially, the study demonstrated that the benefits of dietary restriction were not restricted to innate immune enhancement but also significantly improved responses to checkpoint blockade therapies, such as anti-PD-1 antibodies. This combinatorial approach elicited synergistic effects, suggesting that dietary interventions could sensitize tumors to immunotherapy by empowering the immune cells that these treatments aim to unleash.
The molecular nexus uncovered in this investigation sheds light on the intricate metabolic crosstalk that governs immune cell fate. The interplay between nutrient sensing pathways, energy metabolism, and epigenetic remodeling under dietary restriction conditions converges to create a permissive environment for CD8+ T cell memory differentiation and effector function. This multifaceted regulation underscores the importance of systemic metabolic homeostasis in shaping immune competence against cancer.
Yet, the translational implications of this work extend far beyond cancer immunology. Given that dietary interventions are low-cost, accessible, and comparatively safe, they offer an adjunct or even a preventative strategy to enhance immune surveillance in populations at risk for malignancies. Furthermore, understanding how metabolic states influence immune responsiveness could inform the design of more personalized nutrition-informed therapeutic protocols in oncology clinics.
While the results obtained from preclinical models are compelling, the researchers emphasize the imperative for cautious optimism as human physiology presents additional complexities. Nevertheless, ongoing clinical studies aim to evaluate the feasibility and efficacy of calibrated dietary restriction protocols in cancer patients undergoing immunotherapy, which could validate and refine these findings for broader clinical application.
The mechanistic insights gained also prompt further investigations into the potential role of specific nutrient modifications—such as amino acid depletion or micronutrient supplementation—in fine-tuning T cell responses. Harnessing such tailored nutritional approaches could foster an era of metabolic immunotherapy, wherein diet acts as a primary modulator of treatment outcomes.
Beyond immunotherapy, this research invites exploration into how dietary restriction may influence other immune-mediated diseases, including autoimmune disorders and infectious diseases, by reprogramming T cell subsets and their metabolic profiles. The systemic nature of dietary effects underscores the potential for wide-reaching impacts on immune health.
Moreover, the study sets a precedent for integrating multi-omics analyses—transcriptomics, metabolomics, and epigenomics—to elucidate the comprehensive landscape of immune cell plasticity under metabolic stress. Such high-resolution mapping is vital to unearth novel therapeutic targets and biomarkers predictive of treatment response.
In light of these findings, oncologists and immunologists face an exciting frontier, where dietary strategies could complement pharmacological interventions to harness the full potential of the immune system against cancer. This paradigm shift stresses personalized medicine’s values, integrating lifestyle factors into mainstream treatment algorithms.
The research team also highlighted the importance of temporal dynamics in dietary restriction, noting that the timing and duration of nutritional interventions profoundly affect immune outcomes. This temporal component is a critical factor for optimizing protocols that maximize therapeutic benefits while minimizing potential adverse effects.
In conclusion, this seminal study offers a compelling vision for the future of cancer therapy, where metabolic modulation via dietary restriction emerges as a powerful enhancer of anti-tumor immunity and immunotherapy efficacy. As our understanding deepens, such integrative approaches could redefine standards of care, improving survival and quality of life for patients worldwide.
Subject of Research:
Dietary restriction-induced metabolic reprogramming of CD8+ T cells to enhance anti-tumor immunity and immunotherapy efficacy.
Article Title:
Dietary restriction reprograms CD8+ T cell fate to enhance anti-tumour immunity and immunotherapy responses.
Article References:
Oswald, B.M., DeCamp, L.M., Longo, J. et al. Dietary restriction reprograms CD8+ T cell fate to enhance anti-tumour immunity and immunotherapy responses. Nat Metab (2025). https://doi.org/10.1038/s42255-025-01415-6
Image Credits: AI Generated
DOI:
https://doi.org/10.1038/s42255-025-01415-6