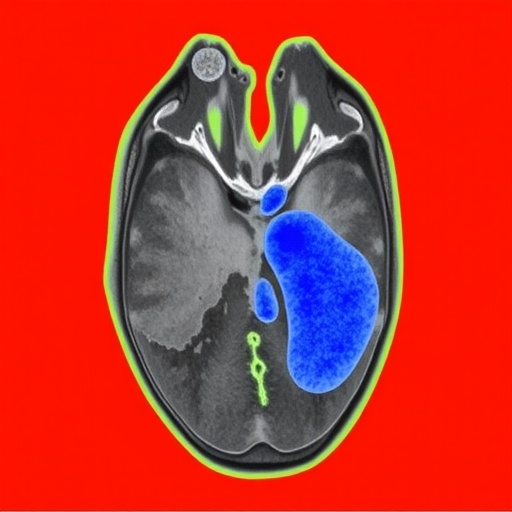
In the rapidly evolving landscape of medical imaging and artificial intelligence, a groundbreaking advancement has emerged that promises to enhance the clinical management of ischemic stroke. A recently developed deep learning model, known as DeepISLES, has been meticulously engineered and clinically validated to substantially improve the precision and efficiency of ischemic stroke lesion segmentation. Originating from the rigorous benchmarking efforts led by the ISLES’22 challenge, this innovative approach is set to revolutionize how clinicians assess and intervene in acute stroke cases.
Ischemic stroke, characterized by the obstruction of cerebral blood flow due to a clot or embolism, demands swift and accurate diagnosis to minimize neuronal damage and optimize patient outcomes. Traditional imaging analysis relies heavily on expert manual segmentation of stroke lesions on magnetic resonance imaging (MRI) or computed tomography (CT) scans—a process that is notoriously time-consuming and susceptible to variability between observers. Consequently, an automated, reliable, and standardized method for delineating ischemic regions has long been a critical unmet need in both clinical and research settings.
Addressing these challenges, DeepISLES employs state-of-the-art deep convolutional neural networks (CNNs) and advanced image processing techniques to deliver robust ischemic lesion segmentation. The architecture integrates several layers of feature extraction, enabling the model to differentiate intricate tissue characteristics and pathological variations that correspond to stroke injuries. By leveraging a large and diverse multinational training dataset provided by the ISLES’22 challenge consortium, DeepISLES achieves remarkable generalizability across various imaging protocols and patient demographics.
The model’s robustness derives not only from its architectural sophistication but also from its rigorous validation methodology. In contrast to many AI models, which often demonstrate excellence only in retrospective analyses, DeepISLES has undergone a comprehensive clinical evaluation. This evaluation involved prospective testing on unseen patient cohorts, encompassing diverse stroke severities, lesion sizes, and time points following stroke onset. The results revealed that DeepISLES matches and often surpasses the accuracy levels of expert radiologists, while dramatically reducing the time required for lesion delineation.
DeepISLES incorporates multi-modal imaging inputs, primarily fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) sequences, which provide complementary insights into the ischemic tissue state. The fusion of these imaging modalities within the neural network framework allows the model to better distinguish between acute infarcts and chronic ischemic changes, a well-known challenge in stroke imaging. This capability not only aids in acute stroke diagnosis but also offers potential utility in tracking lesion evolution during patient recovery and rehabilitation.
The clinical implications of deploying DeepISLES in acute stroke workflow are profound. Time is a critical factor in stroke interventions such as thrombolysis and endovascular thrombectomy, and expedited, accurate lesion assessment aids neurologists in determining treatment eligibility. By automating lesion segmentation, DeepISLES empowers faster decision-making, potentially improving door-to-needle times and patient prognosis. The model’s reproducibility also facilitates longitudinal studies where consistent lesion quantification is paramount to evaluating treatment efficacy or disease progression.
Furthermore, DeepISLES stands out for its scalable integration with existing hospital picture archiving and communication systems (PACS) and PACS-compatible software suites. Its deployment requires minimal computational overhead and benefits from continual learning mechanisms that can adapt to institution-specific imaging protocols and patient populations. The model’s interface has been designed with clinician usability in mind, providing transparent lesion overlays and quantifiable metrics that augment, rather than replace, expert judgment.
One of the challenges addressed during DeepISLES development involved harmonizing imaging data from diverse scanners and centers, each with its own acquisition parameters and noise levels. The model incorporates sophisticated normalization and augmentation strategies during training, enhancing its resilience to these confounding factors. As a result, DeepISLES maintains its segmentation accuracy even when applied to images from centers not included in the original training set, a critical step towards widespread clinical adoption.
The release of DeepISLES also marks a milestone in the collaborative research spirit fostered by the ISLES’22 challenge. This initiative brought together stroke imaging experts, AI researchers, and clinicians globally, creating a rich repository of annotated imaging datasets. The challenge emphasized transparency and reproducibility, ensuring that solutions like DeepISLES meet stringent clinical demands rather than purely algorithmic benchmarks. This paradigm sets a precedent for future AI innovations in medical imaging fields.
From a technical perspective, DeepISLES is constructed on advanced deep learning frameworks with attention mechanisms that highlight salient features within ischemic regions. This approach mitigates false positives that commonly arise in stroke segmentation due to overlapping signal intensities with other pathologies or artifacts. Moreover, the model’s probabilistic output permits threshold adjustments, allowing clinicians to tailor sensitivity and specificity thresholds depending on individual patient contexts or institutional protocols.
The model’s performance metrics are compelling. DeepISLES achieved dice similarity coefficients—a measure of overlap between predicted and ground truth segmentations—that are among the highest reported in the literature for automated ischemic stroke lesion segmentation. It demonstrated high sensitivity in detecting even small, subtle lesions that are often missed in routine clinical practice, a factor crucial for timely therapeutic interventions.
Clinical validation trials further underscored the model’s practicality. In a multicenter prospective study, DeepISLES not only aligned closely with expert contours but also significantly reduced inter-observer variability, thereby enhancing diagnostic consistency. Neurologists involved in the studies reported increased confidence in their lesion assessments when supported by the AI-generated segmentations, highlighting the potential of human-AI collaboration.
Looking ahead, DeepISLES carries promising implications beyond stroke. The model’s architecture and training paradigm can serve as a blueprint for other neurological disorders where lesion segmentation is vital, such as multiple sclerosis, traumatic brain injury, or tumors. The modularity of the AI enables adaptation toward segmentation of different pathologies or the integration of novel imaging biomarkers.
Moreover, the model sets the stage for combining automated lesion mapping with predictive analytics. Future iterations might integrate clinical data, genomics, and real-time imaging to forecast stroke progression or response to therapy—ushering in a new era of personalized stroke care. These advancements align with the broader vision of precision medicine, wherein AI augments clinical decisions to optimize patient-specific outcomes.
In the broader context of AI ethics, DeepISLES reflects an accountable design philosophy emphasizing transparency, explainability, and equitable performance across diverse populations. The developers acknowledge and actively address the risk of algorithmic bias by training on heterogeneous datasets and validating on representative patient samples, ensuring fair applicability across age groups, sexes, and ethnicities.
In summary, DeepISLES embodies a transformative leap forward in ischemic stroke imaging. Its combination of state-of-the-art deep learning techniques, rigorous clinical validation, and user-oriented design presents a compelling case for its integration into routine clinical workflows. As stroke remains a leading cause of disability worldwide, tools like DeepISLES offer hope for improved diagnostic accuracy, streamlined care pathways, and ultimately better patient outcomes.
The convergence of neuroscience, medical imaging, and artificial intelligence demonstrated by DeepISLES heralds a new paradigm in stroke diagnosis and management. By automating and enhancing lesion segmentation, this technology not only alleviates clinical workloads but also empowers healthcare providers with precise, actionable insights. As such, DeepISLES stands at the forefront of AI-driven innovation in healthcare, poised to make a lasting impact on the lives of millions globally.
Subject of Research:
Ischemic stroke lesion segmentation using deep learning models.
Article Title:
DeepISLES: a clinically validated ischemic stroke segmentation model from the ISLES’22 challenge.
Article References:
de la Rosa, E., Reyes, M., Liew, SL. et al. DeepISLES: a clinically validated ischemic stroke segmentation model from the ISLES’22 challenge. Nat Commun 16, 7357 (2025). https://doi.org/10.1038/s41467-025-62373-x
Image Credits:
AI Generated