In an unprecedented study published by researchers at the forefront of pharmacology and toxicology, the mechanism of cisplatin-induced toxicity in the hippocampus has been explored in a comprehensive and revealing manner. This impactful research has brought to light critical insights into the effects of cisplatin, a widely used chemotherapy agent, particularly regarding its neurotoxic effects at varying doses. The hippocampus, a vital region in the brain associated with memory formation and learning, proves to be significantly impacted by cisplatin treatment. Understanding the dose-dependent nature of its toxicity is paramount for both clinicians and patients undergoing cancer treatment.
Cisplatin, a platinum-based compound, has been a cornerstone in the treatment of various malignancies, including testicular, ovarian, and lung cancers. While its efficacy in targeting and killing cancer cells is well-documented, the collateral damage it induces on non-cancerous tissues raises significant concerns. This research underlines the implications of such toxicity in the context of long-term cognitive and neural health. Patients receiving cisplatin could experience subtle yet significant neurological side effects, potentially leading to debilitating conditions that affect quality of life, further complicating cancer recovery and survival.
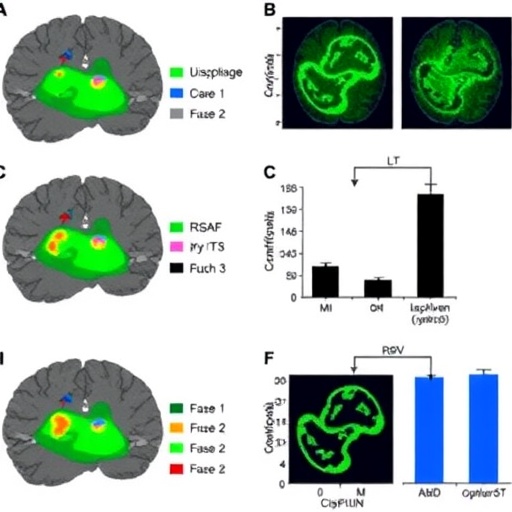
The study conducted by Altunkaya, Ateş, and Bulut employed a sophisticated methodology that involved administering cisplatin to animal models, allowing researchers to observe the resulting neurotoxic effects in the hippocampus closely. One of the critical findings of this research was the identification of a dose-dependent mechanism where lower doses may produce minimal effects, while higher doses resulted in pronounced damage. This insight has critical implications for developing better treatment protocols that could mitigate the neurotoxic impacts of cisplatin on patients undergoing chemotherapy.
Furthermore, the research delves into the cellular and molecular pathways activated by cisplatin toxicity. It has been shown that exposure to higher doses of cisplatin leads to increased levels of oxidative stress, inflammation, and apoptosis in hippocampal neurons. The results indicate that cisplatin not only impacts neuronal viability but may also alter synaptic function and plasticity, further exacerbating cognitive impairment. These revelations emphasize the urgent need for neuroprotective strategies during cisplatin treatment to safeguard cognitive functions in patients.
In addition to the direct cellular effects, the study also highlights the importance of understanding the pharmacokinetics of cisplatin. The concentration of the drug in the bloodstream, its distribution in various tissues, and its clearance rate can significantly influence its toxicity profile. Knowledge of these parameters can aid healthcare professionals in adjusting treatment regimens, potentially lowering the risk of neurological damage while still effectively combating cancerous cells.
Another notable aspect of this research is its focus on the long-term implications of cisplatin-induced toxicity. As cancer treatments extend and evolve, it becomes increasingly vital to consider the adverse effects that persist even after therapy has concluded. Cognitive decline and neurodegeneration are significant concerns for survivors, particularly as they age. This study sets a precedent for further exploration into post-treatment care and the implementation of cognitive assessments for cancer survivors.
Moreover, the study suggests that there may be potential for pharmacological interventions aimed at reducing the neurotoxic effects of cisplatin. By targeting the specific pathways induced by cisplatin, researchers may develop adjuvant therapies that can be administered alongside chemotherapy. These therapies would ideally protect the hippocampal region from cisplatin-induced damage, preserving cognitive function and improving overall quality of life for patients.
Additionally, beyond the experimental framework, these findings can reverberate through public health policy and patient care strategies. As the rankings of cancer drugs focus heavily on efficacy against neoplasms, it is vital to take into account the broader impact of such treatments on mental health and neurological well-being. The integration of neurotoxicity markers into clinical assessments could enable earlier interventions and better management of side effects associated with potent chemotherapeutics.
The repercussions of this study extend far beyond the laboratory. Awareness of the neurotoxic potential of cisplatin must lead to proactive conversations between oncologists and patients regarding the benefits and risks associated with its use. Clinician-patient dialogue should encompass not only the likelihood of cancer remission but also the potential cognitive impairments that could emerge, fostering a more holistic approach to cancer care.
With the increasing incidence of cancer globally, there is a pressing need for ongoing research in the field of oncology that does not only examine tumor responses but also addresses the comprehensive wellbeing of patients. As therapy regimens evolve with the introduction of newer, less neurotoxic drugs, understanding the legacy of older agents like cisplatin remains critical. The findings from this pivotal research serve as an important reminder of the importance of balancing treatment efficacy with patient quality of life.
As researchers and healthcare professionals digest the implications of this study, the quest for safer, more effective cancer therapies continues. Lessons learned from cisplatin toxicity could inform future drug development strategies, leading to more refined approaches that prioritize patient health holistically. The ultimate goal must be to ensure that as patients fight against cancer, they are not also waging an unwitting battle against cognitive decline.
This research stemming from the examination of cisplatin-induced toxicity is a clarion call for the scientific community. Neuroscience, toxicology, and oncology must intersect to address the multifaceted impacts of cancer treatment. As the body of evidence grows, so too should the commitment to improved patient care, where factors like cognitive health are placed at the forefront alongside cancer treatment efficacy.
In conclusion, the journey of understanding cisplatin-induced toxicity in the hippocampus ushers in a new era of integrative treatment planning and gives rise to the imperative need for research that prioritizes the patient experience during and after cancer therapy. Continued investigation in this area promises to yield breakthroughs not just in the fight against cancer but in fostering long-term cognitive health for survivors, making every effort toward mitigating the collateral damage of chemotherapy a worthwhile pursuit.
Subject of Research: Neurotoxic effects of cisplatin in the hippocampus
Article Title: Cisplatin-induced toxicity in the hippocampus: a dose-dependent mechanism of damage
Article References:
Altunkaya, M., Ateş, M.B., Bulut, A. et al. Cisplatin-induced toxicity in the hippocampus: a dose-dependent mechanism of damage.
BMC Pharmacol Toxicol (2025). https://doi.org/10.1186/s40360-025-01050-7
Image Credits: AI Generated
DOI: 10.1186/s40360-025-01050-7
Keywords: Cisplatin, neurotoxicity, hippocampus, dose-dependent mechanism, chemotherapy, cognitive health, cancer treatment.