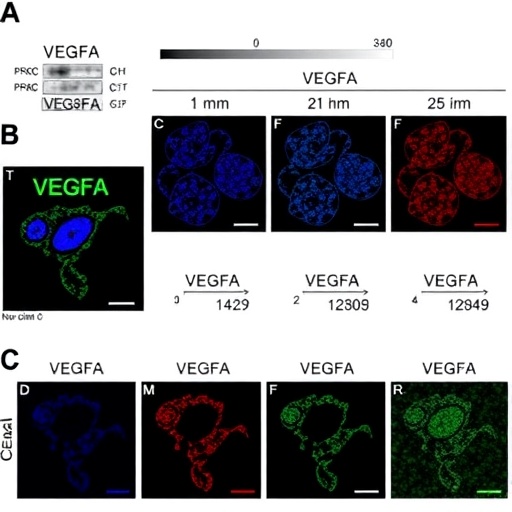
In a groundbreaking study published in the Journal of Ovarian Research, researchers Qin, Zhang, Yin, and their team have unveiled the pivotal role of circVEGFA in regulating apoptosis within porcine ovarian granulosa cells. Their findings provide insight into the complex molecular interactions that underpin ovarian health and dysfunction, potentially opening up new avenues for therapeutic interventions in reproductive biology. The unique circular RNA, circVEGFA, has been identified as a key modulator of cellular processes crucial for ovarian function.
The study is particularly significant in the context of female fertility, as granulosa cells play an essential role in the maturation of oocytes and the overall health of the ovarian environment. This research highlights the intricate mechanisms governing granulosa cell survival and how disruptions in these pathways can contribute to reproductive challenges. The authors have meticulously documented how circVEGFA interacts with microRNA-21-3p, a critical regulator of cell fate decisions, ultimately leading to reduced apoptosis in these vital cells.
In their methodology, the researchers employed a range of advanced techniques, including RNA sequencing, to profile the expression of circVEGFA and its target pathways in porcine models. They demonstrated that circVEGFA acts as a molecular sponge, binding to miR-21-3p, which is known to promote cell death when unregulated. This interaction not only stabilizes circVEGFA but also ensures a robust expression of TMX4, a gene linked to cell survival and stress response.
The implications of these findings extend beyond porcine models, suggesting that similar mechanisms may operate in other mammalian systems, including humans. By elucidating the relationship between circVEGFA and miR-21-3p, this research underlines the potential for developing targeted therapies aimed at enhancing ovarian function and preserving female fertility. As circRNAs are known for their stability and abundance in various tissues, they represent a promising new class of biomolecules for therapeutic development.
The study also delves into the broader implications of circVEGFA in reproductive physiology, raising questions about its potential role in ovarian follicle development and the survival of oocytes. These insights could lead to a deeper understanding of conditions that affect fertility, such as polycystic ovary syndrome (PCOS) and premature ovarian insufficiency (POI). As researchers continue to decode the regulatory networks in granulosa cells, they may uncover novel strategies to combat these prevalent reproductive disorders.
Moreover, the findings invite further exploration into the potential of circRNAs as biomarkers for reproductive health. Given their specific expression profiles and their roles in regulating key cellular processes, circRNAs could serve not only as indicators of ovarian health but also as targets for future diagnostics and therapeutics. The promise of precision medicine in reproductive health hinges on such discoveries, which could revolutionize how we approach fertility treatments.
As the field of epitranscriptomics continues to expand, the relevance of circular RNAs cannot be overlooked. The landscape of RNA biology is rapidly evolving, and circVEGFA’s demonstrated influence on apoptosis adds another layer of complexity to our understanding of gene regulation. The research team’s work sheds light on a previously underappreciated aspect of RNA functionality and its implications for cell survival.
Future investigations are likely to focus on the mechanistic pathways through which circVEGFA exerts its effects on TMX4 expression and cell viability. Understanding the signaling cascades involved, as well as the interaction with other non-coding RNAs, will be critical in piecing together the multifaceted roles of circRNAs in ovarian biology. This research not only paves the way for advancing scientific knowledge but also underscores the vital importance of collaborative efforts in unraveling the intricacies of reproductive health.
The study has sparked excitement within the scientific community, prompting discussions and reflections on the future of ovarian research. As insights into the molecular underpinnings of granulosa cell function deepen, the potential for translational applications grows. Researchers are hopeful that findings such as those presented by Qin and colleagues will inspire further studies that bridge the gap between basic science and clinical application in reproductive medicine.
In conclusion, this pioneering research highlights circVEGFA as a crucial player in the maintenance of porcine ovarian granulosa cell survival. By demonstrating its regulatory role and interaction with miR-21-3p, the study opens up new possibilities for therapeutic interventions targeting fertility issues. As interest in circRNAs continues to surge, the door is wide open for innovative research that could reshape our understanding of reproductive health and disease.
Subject of Research: The role of circVEGFA in apoptosis regulation in porcine ovarian granulosa cells.
Article Title: circVEGFA inhibits apoptosis in porcine ovarian granulosa cells by binding to miR-21-3p and up-regulating TMX4 expression.
Article References:
Qin, X., Zhang, J., Yin, C. et al. circVEGFA inhibits apoptosis in porcine ovarian granulosa cells by binding to miR-21-3p and up-regulating TMX4 expression.
J Ovarian Res 18, 155 (2025). https://doi.org/10.1186/s13048-025-01738-8
Image Credits: AI Generated
DOI:
Keywords: circVEGFA, granulosa cells, apoptosis, miR-21-3p, TMX4, ovarian function, infertility, circular RNAs, reproductive health.