In recent scientific endeavors, the intersection of pharmacology and dietary research has yielded impressive results, particularly regarding obesity management. A groundbreaking study, led by Naidu B. Ommi and Sailendra N. Nichenametla from the Orentreich Foundation for the Advancement of Science Inc., uncovers the potential of buthionine sulfoximine (BSO) as a proxy for the sulfur amino acid restriction (SAAR) diet, which has long been touted for its health benefits, including significant fat loss and weight management. Published in the esteemed journal Aging (Aging-US), this innovative research may offer a new avenue for the treatment of obesity—one that sidesteps the arduous dietary constraints that often dissuade compliance among individuals.
Understanding the SAAR diet’s formulation is crucial for appreciating the groundbreaking implications of this study. The SAAR diet is characterized by a notable restriction of sulfur-containing amino acids, specifically methionine and cysteine. This particular dietary regime has demonstrated remarkable health benefits in rodent models, notably longevity enhancements and significant reductions in body fat. Adherence to such a restrictive diet poses challenges for human subjects due to its strict nature. Consequently, researchers have sought alternatives that emulate the SAAR benefits without the dietary burdens.
In their exploration, the research team turned to BSO, a compound recognized for its ability to lower glutathione (GSH) levels within the body. GSH is a vital antioxidant composed of three amino acids, including cysteine, which plays an essential role in cellular detoxification and redox reactions. The presumption behind using BSO hinges on its potential to mimic the biochemical effects of SAAR, thereby circumventing the need for dietary restrictions. The advantage of utilizing BSO lies in its simplicity as a pharmacological approach, paving the way for compliance among individuals who may struggle to adhere to convoluted dietary regimens.
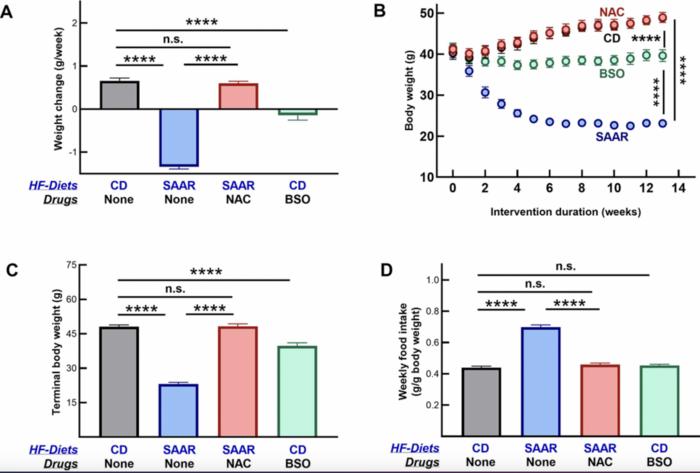
To discern the efficacy of BSO as a surrogate for the SAAR diet, the researchers conducted an elaborate study involving four groups of eighteen-week-old male C57BL/6NTac mice. The mice were fed either a high-fat diet with varying levels of methionine, the SAAR diet with further supplementation, or a regular diet augmented with BSO. What unfolded was a striking revelation: mice treated with BSO evidenced reductions in fat mass that closely mirrored those observed in subjects subjected to the SAAR diet. Furthermore, the BSO-treated cohort demonstrated a striking ability to prevent weight gain without any corresponding decrease in food intake or muscle mass—significant findings that underline the unique potential of this pharmacological approach.
Interestingly, the mechanisms underlying the weight management effects of BSO and the SAAR diet diverged at the organ level. The SAAR diet appeared to exert its beneficial effects primarily through modifications in liver metabolism, influencing pathways associated with fat storage and energy expenditure. Conversely, BSO acted more prominently within renal tissues, suggesting a more nuanced metabolic interplay at work. In both instances, increases in serum serine levels were observed, hinting at an intricate biochemical relationship between amino acid metabolism and adiposity.
The implications of these findings extend beyond mere curiosity; they present tangible opportunities in combating obesity-related metabolic disorders. As obesity serves as a precursor to numerous chronic conditions—most notably cardiovascular diseases, type 2 diabetes, and neurodegenerative disorders—effective interventions are paramount. The pharmacological adoption of BSO stands to revolutionize the approach to obesity management, especially among populations with documented adherence challenges to dietary restrictions.
Another compelling aspect of this research resides in its evaluation of safety concerns surrounding BSO. Over a 13-week study period, no significant signs of liver or kidney toxicity were reported, further strengthening the argument for BSO’s applicability in human populations. This safety profile bolsters confidence in its repurposing as an anti-obesity agent, as it has previously traversed human clinical trials for distinct medical conditions, potentially streamlining the approval process for novel applications in metabolic health.
Nevertheless, the researchers maintain a discerning caution, acknowledging the necessity for further exploratory studies. While the results herald promise, additional work is required to evaluate the long-term effects of BSO on metabolic health in both animal models and human trials. It serves as a reminder that while pharmacological alternatives can provide convenience and efficacy, comprehensive assessments are essential to ensure safety and effectiveness.
The study’s results resonate with the notion that managing body weight can be more nuanced than merely adhering to caloric restrictions. By uncovering the biochemical intricacies at play between dietary components and pharmacological interventions, Ommi and Nichenametla have opened new doors for understanding obesity’s metabolic underpinnings. With obesity rates climbing dramatically worldwide, innovative solutions are urgently needed—BSO could emerge as a critical component in addressing this global health crisis.
In conclusion, the novel findings from the study conducted by Ommi and Nichenametla are not only illuminating the pathways through which BSO operates but also highlighting a paradigm shift in how obesity treatment could be approached in the future. By potentially allowing individuals to achieve significant health benefits without compromising their dietary preferences, this research lays the groundwork for new methodologies in obesity and metabolic disorder treatment. The prospect of managing obesity pharmacologically rather than through stringent diet regimens is an exciting development in nutritional science with significant implications for public health and individual patient outcomes.
Subject of Research: Buthionine sulfoximine (BSO) as an alternative to sulfur amino acid restriction (SAAR) for obesity treatment.
Article Title: Pharmacological recapitulation of the lean phenotype induced by the lifespan-extending sulfur amino acid-restricted diet.
News Publication Date: May 13, 2025.
Web References: Aging-US Journal Article.
References: doi:10.18632/aging.206237.
Image Credits: Copyright: © 2025 Ommi et al.