In the relentless quest to mitigate climate change, the capture and removal of atmospheric carbon dioxide remain paramount challenges. Existing carbon capture methods often grapple with a fundamental efficiency paradox: chemical compounds that excel at absorbing CO₂ tend to release it slowly, while those that facilitate rapid release capture CO₂ less effectively. This inherent tradeoff has stymied efforts to optimize direct air capture technologies at the scale required to meaningfully curb rising global carbon levels.
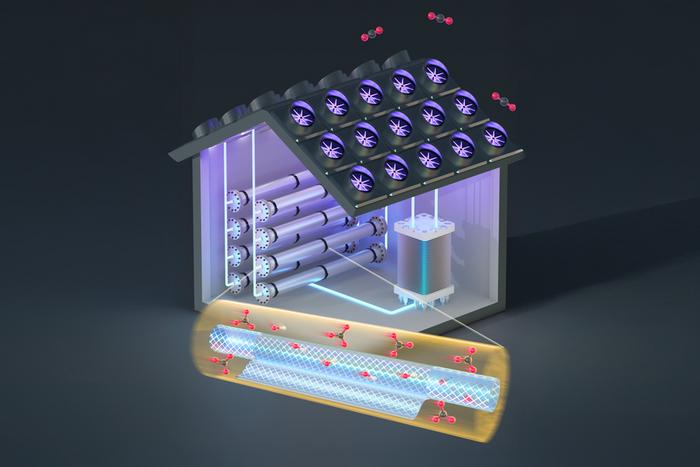
Researchers at the Massachusetts Institute of Technology (MIT) have ingeniously circumvented this limitation by introducing an innovative intermediate stage leveraging nanoscale filtering membranes. Their breakthrough strategy separates critical ionic species—carbonate and hydroxide ions—within the absorption and release cycles, allowing each stage to operate independently at optimal efficiency. This advancement represents a sixfold improvement in electrochemical CO₂ capture and release performance, while simultaneously reducing operational costs by more than 20%.
The new approach, detailed in a recent publication in ACS Energy Letters, was crafted by MIT doctoral candidates Simon Rufer, Tal Joseph, and Zara Aamer, alongside mechanical engineering professor Kripa Varanasi. Their work confronts the bottleneck caused by a shared aqueous solution where both CO₂ absorption and release reactions occur. Typically, the absorption stage demands a solution rich in hydroxide ions to chemically capture CO₂ as carbonate, whereas the release stage requires a high carbonate concentration to regenerate gaseous CO₂. The incompatibility of these ionic environments has long limited overall system efficiency.
To resolve this, the MIT team inserted a nanofiltration system between the absorption and release phases. This membrane selectively distinguishes ions based on their electric charge: carbonate ions carry a charge of minus two, while hydroxide ions have a charge of minus one. This charge differential enables the membrane to separate the two species with approximately 95% efficiency under conditions mimicking real-world operation. By segregating these ions, the system recycles hydroxides back to the absorption step and delivers carbonates to the release stage, permitting simultaneous optimization without chemical compromise.
This ion separation is critically important because, during electrochemical CO₂ release, protons are introduced to convert carbonate ions back into carbon dioxide and water. If hydroxide ions coexist in significant amounts, they readily neutralize the protons, forming water and hindering CO₂ liberation. The MIT team demonstrated that without effective ion separation, this undesired reaction drastically suppresses the system’s ability to extract CO₂. The nanofiltration membrane thus not only enhances CO₂ output but also stabilizes the electrochemical cell’s functionality.
Quantitative techno-economic modeling further substantiated the practical benefits of this development. Conventional systems currently capture carbon at a cost upwards of $600 per ton. Incorporating nanofiltration reduces this cost to approximately $450 per ton, marking a significant stride toward economic viability. Moreover, the innovated system demonstrates a broader operational tolerance, maintaining high efficiency despite fluctuations in ion concentrations—a prevalent challenge in scalable systems that operate "on a knife’s edge."
Beyond direct air capture applications, the MIT team’s concept holds promise for point-source emissions facilities, such as power plants, where concentrated CO₂ streams demand efficient sequestration solutions. Additionally, the membrane-enabled separation strategy could be adapted to downstream processes that chemically convert captured CO₂ into valuable fuels and feedstocks, overcoming similar ionic tradeoffs hampering reaction rates and yields.
Another compelling advantage of this technology lies in enabling safer, environmentally benign absorbents. Many current sorbents possess toxicity or environmental persistence issues. By enhancing reaction rates through efficient ion management, the process expands the palette of viable chemicals, allowing the use of safer compounds that would otherwise be hampered by slower absorption kinetics.
The research team stresses that their solution is deployable using commercially available components, facilitating straightforward retrofitting to existing carbon capture installations. This modularity is crucial for accelerating adoption across diverse industries and infrastructure scales. Further optimization and continued cost reductions could push capture expenses near $200 per ton, a threshold likely to catalyze widespread deployment.
Simon Rufer emphasizes the immediacy of market opportunities, noting that carbon credits currently transact at prices exceeding $500 per ton. Their projected cost reductions not only promise enhanced commercial competitiveness but may also increase the breadth of buyers qualified to invest in carbon offsets, supporting faster decarbonization pathways worldwide.
Professor Varanasi highlights the broader vision driving this work: “We need to think about scale from the get-go when it comes to carbon capture, as making a meaningful impact requires processing gigatons of CO₂.” This mindset has fueled their pursuit of system-level innovations, focused on practical, scalable solutions that balance chemistry, engineering, and economics.
Their findings stand as a beacon of innovation in the quest to balance CO₂ absorption and release, demonstrating how nanoscale engineering can unlock new efficiencies in climate technology. With support from Shell International Exploration and Production, the MIT Energy Initiative, and the U.S. National Science Foundation, this work leverages cutting-edge facilities at MIT.nano to advance carbon capture science into viable, impactful technology.
As climate change accelerates, breakthroughs like this one offer a critical bridge between laboratory insight and large-scale implementation. By enabling existing CO₂ capture systems to operate more efficiently and cost-effectively, such advances bring the global community steps closer to meeting urgent decarbonization goals and safeguarding planetary health.
Subject of Research: Carbon dioxide capture and electrochemical release efficiency enhancement using nanoscale membrane filtration
Article Title: "Carbonate/Hydroxide Separation Boosts CO2 Absorption Rate and Electrochemical Release Efficiency"
Web References:
- Journal link: http://dx.doi.org/10.1021/acsenergylett.5c00893
- Article DOI: 10.1021/acsenergylett.5c00893
References:
Rufer, S., Joseph, T., Aamer, Z., & Varanasi, K. (2023). Carbonate/Hydroxide Separation Boosts CO2 Absorption Rate and Electrochemical Release Efficiency. ACS Energy Letters. http://dx.doi.org/10.1021/acsenergylett.5c00893
Image Credits: Courtesy of Kripa Varanasi, Simon Rufer, Tal Joseph, and Zara Aamer
Keywords
Carbon capture, carbon sequestration, chemical engineering, electrochemical CO₂ release, nanofiltration membrane, environmental technology, climate change mitigation, sustainability, pollution reduction, carbon emissions, electrochemical cell efficiency, direct air capture