A functional, healthy bladder is something that many of us take for granted. Yet millions of Americans deal with bladder issues, ranging from temporary inconveniences to long-lasting conditions. While many bladder disorders can be managed with non-invasive solutions, some conditions may require surgery to restore bladder function.

Credit: Mark Seniw at Northwestern University, Simpson Querrey Institute
A functional, healthy bladder is something that many of us take for granted. Yet millions of Americans deal with bladder issues, ranging from temporary inconveniences to long-lasting conditions. While many bladder disorders can be managed with non-invasive solutions, some conditions may require surgery to restore bladder function.
In patients with major bladder issues, a cystectomy may need to be performed. In this procedure, some or all of the bladder is removed (reasons for this may include acute trauma or bladder cancer). Sometimes, to compensate for the loss of tissue, the bladder is augmented (made larger), typically with a piece of bowel tissue from the patient. Bladder augmentation can also be performed among patients with spinal cord injuries or with congenital conditions such as spina bifida or bladder exstrophy.
While bladder augmentation can greatly improve a patient’s quality of life, the surgery has a high risk of complications, and patients need to be extensively monitored for months after the procedure. Thousands of bladder surgeries are performed every year in the U.S., yet significant improvements in bladder augmentation and monitoring have not been seen in many decades.
A collaborative NIH-funded team at Northwestern University is working to change that. They are tackling the issues from two different vantage points: improving bladder function by utilizing a cell-seeded, biodegradable construct that both augments the bladder and facilitates tissue regeneration, along with enhancing patient monitoring by developing an implantable bladder sensor. Their research, many years in the making, has reached an important clinical milestone—evaluating their technologies in non-human primate models.
“As biotechnologies advance, they will need to integrate more seamlessly with the human body,” said Jessica Falcone, Ph.D., a program director in the Division of Discovery Science and Technology at the National Institute of Biomedical Imaging and Bioengineering (NIBIB). “This research team has successfully navigated interfacing with the bladder without interfering with its function, bridging the fields of tissue regeneration and flexible bioelectronics. Such advances could enhance the lives of future patients requiring bladder surgery.”
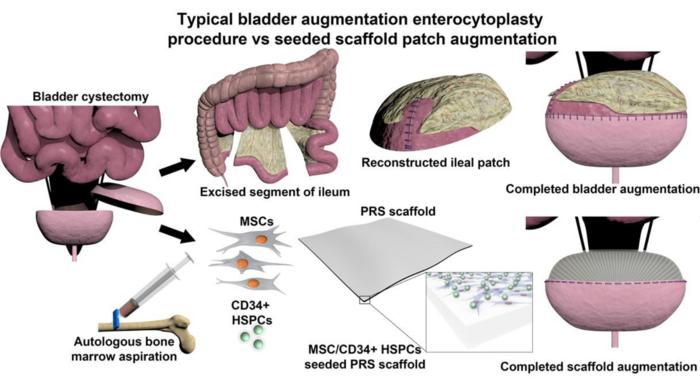
Building a better bladder substitute
Bladder augmentation surgery has been documented for over a century, but the procedure wasn’t performed routinely until after 1950. Frequently, a segment of the ileum (part of the small intestine) is removed and added to the bladder to make it larger. But these two types of tissues are quite different from each other—the bladder is an excretory organ, while the intestine is an absorbative one.
“While using bowel tissue for bladder augmentation has been the gold standard for many decades, it is fraught with numerous clinical complications,” said study author Arun Sharma, Ph.D., a professor at Northwestern University and Director of Pediatric Urological Regenerative Medicine and Surgical Research at the Lurie Children’s Hospital and Stanley Manne Children’s Research Institute. “The two tissues have anatomical and physiological incompatibility, leaving the patient with a reconstructed bladder that is prone to dysfunction, infection, and failure.”
Instead of using ileum to patch the bladder, Sharma and his colleagues explored a different option: using a synthetic construct that helps the native bladder tissue to regrow. A scaffold seeded with autologous stem cells can facilitate bladder tissue growth while also physically augmenting the bladder. After a time, the scaffold biodegrades, leaving behind only regenerated bladder tissue. Their research was recently reported in the journal PNAS Nexus.
Scaffolds to facilitate bladder regeneration have been previously developed, yet none have ultimately been successful after clinical evaluation. In this study, the researchers turned to citric acid, a naturally occurring molecule that can be easily crosslinked with other materials to affect its physical and chemical properties. Study co-author Guillermo Ameer, Sc.D., Director of the Center for Advanced Regenerative Engineering at Northwestern University, has previously reported biodegradable citrate-based scaffolds for musculoskeletal applications, but this biomaterial needed to be tweaked for use as a bladder substitute.
“The scaffold needed to mimic the soft and elastic properties of the bladder, but it also needed to be suture-able and effectively hold urine,” explained Ameer. “By precisely controlling the degree of crosslinking of the scaffold, we could alter its stiffness while maintaining its elasticity and biodegradability, all while facilitating the growth of the seeded stem cells to regenerate the bladder tissue.”
The research team evaluated their cell-seeded biomaterial in a baboon model, augmenting bladders with either the biodegradable scaffold or ileum. After monitoring the baboons for two years, they found that animals treated with the biodegradable scaffold had markedly reduced clinical complications (including urinary tract infections, kidney swelling, and vesicoureteral reflux) compared with animals treated with native ileum. What’s more, when the researchers analyzed the regenerated bladder tissue, they found that its composition mirrored that of normal bladder tissue—that is, equal ratios of muscle and collagen and other physiological features. The ileum, on the other hand, is mostly made of muscle tissue, which is a mechanical mismatch for bladder tissue.
“We found that when we seed our scaffold with a population of bone marrow-derived stem cells, the cells take over the graft and simulate native bladder tissue,” explained Sharma. “From a histological and functional standpoint, we found that the regenerated tissue and the native bladder are indistinguishable.”
And importantly, the team’s biomaterial outperformed ileum, which is routinely used for bladder augmentation in humans. “This study is highly translational,” said Sharma. “Anatomically and physiologically, there is no better existing model for the human bladder. This could really set the stage going forward into clinical trials.”
A smart sensor to monitor bladder function
After a patient has bladder surgery, the function of their bladder needs to be monitored to ensure that the organ is working properly. This is usually accomplished through urodynamic testing, a somewhat invasive procedure that can provide information about the bladder, like its ability to hold and empty urine effectively. In addition to being uncomfortable, urodynamic tests must be performed at a doctor’s office, providing only a snapshot of how the bladder is functioning at one specific timepoint.
A potential alternative to urodynamic testing would be to implant a sensor at the time of bladder surgery. This sensor would wirelessly transmit key information, such as when the bladder expands and contracts, which could be used by physicians to remotely monitor bladder function. Such a sensor could immediately notify a clinician of a potential bladder complication, expediting a patient’s care and ultimately improving overall outcomes.
Sharma, Ameer, and colleague John Rogers, Ph.D., Director of the Querrey Simpson Institute for Bioelectronics at Northwestern University, have brought an implantable bladder sensor one step closer to the clinic. Their device, evaluated in baboons and recently reported in the journal PNAS, successfully monitored bladder function for eight weeks without any observed adverse effects, suggesting that this technology could someday be a viable replacement for urodynamic testing.
“This platform gives us the ability to continuously monitor the bladder over long periods of time, supplying very similar information that standard urodynamic testing provides,” said Sharma.
The real-time component of this device is the major advance—it allows clinicians to take timely actions, Sharma said. In a traditional scenario, a patient might suspect that they have a bladder issue, and then schedule urodynamic testing (which may not be available for weeks), and then wait for the results to be interpreted, which ultimately delays their care, he explained. “Our technology could turn that paradigm on its head,” Sharma said.
The sensor is composed of three main parts: a strain gauge, which senses when the bladder expands; a base station, which contains a battery and a Bluetooth chip to wirelessly transmit information; and a helical coil wire that connects these two components. Data from the strain gauge can be used to calculate bladder pressure, which can indicate bladder dysfunction, impending urinary tract issues, and subsequent kidney failure, which can be life-threatening.
The research team evaluated their platform in a baboon model, analyzing the sensor’s performance in an animal with a normal bladder and in an animal with a partial cystectomy. The device wirelessly transmitted bladder data for eight weeks (the duration of the study), allowing for continuous estimation of bladder pressure. As expected, the animal with a partial cystectomy had a higher difference in bladder pressure as compared with the control animal (as a smaller bladder must expand more to hold the same amount of urine).
“Our results suggest that our platform could remotely monitor bladder pressure in patients receiving bladder surgery and in patients recovering from partial cystectomy,” said Sharma. What’s more, all the components of the monitoring platform can be designed to biodegrade, meaning that a patient would not require a second surgery to remove the sensor.
“Because a patient needs to be monitored for roughly 3-6 months after bladder surgery, a short-term, biodegradable sensor would be ideal in this population,” said Ameer. “Bioresorbable platforms have been previously described by our group, and we found that a biodegradable version of our strain gauge completely dissolved under simulated physiological conditions.”
Bringing bladder technology to patients
The authors noted that both of these studies represent key advances in regenerative research, and that the sensor technology is an exciting step forward in the bioelectronics space, as this platform can provide continuous information about the bladder—and the performance of the implantable sensor.
“From a manufacturing standpoint, companies want to know if their device is performing as expected, and a smart electronic device has the capacity to provide this type of information,” said Ameer. “The field of regenerative bioelectronics will help a lot of different stakeholders, from manufacturers, to clinicians, to patients. And most importantly, smart regenerative systems will help patients to make decisions based on their own needs and their own data.”
Ameer also noted that preclinical and clinical research is only the first step in bringing this type of technology to patients. “That’s one of the challenges that we all have, trying to bridge the gap between a publication and a product, which typically requires the involvement of industry or philanthropy along with FDA approval,” he said. “Even with these advancements, these technologies must still be studied further to make sure they are safe and effective in people.”
The studies in this highlight were supported by grants from NIBIB (R01EB026572, T32EB031527) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK109539).
This science highlight describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process—each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research.
Study references:
Matthew I Bury et al. Multipotent bone marrow cell–seeded polymeric composites drive long-term, definitive urinary bladder tissue regeneration, PNAS Nexus, Volume 3, Issue 2, February 2024,
Jihye Kim et al. A wireless, implantable bioelectronic system for monitoring urinary bladder function following surgical recovery, PNAS, Volume 121, Issue 14, February 2024,
Journal
Proceedings of the National Academy of Sciences