In a groundbreaking advance poised to reshape pediatric oncology, researchers have delineated the intricate molecular pathways through which a novel tyrosine kinase inhibitor, TP-0903, induces apoptosis in neuroblastoma cells. This recent discovery elucidates a finely tuned mechanism involving reactive oxygen species (ROS) generation and the modulation of microRNA-mediated gene expression, highlighting a sophisticated interplay that could herald new therapeutic strategies against one of the most aggressive childhood cancers.
Neuroblastoma, a malignancy originating from neural crest elements of the sympathetic nervous system, remains a formidable challenge due to its heterogeneous nature and frequent resistance to conventional treatments. Current therapies, including chemotherapy, radiation, and surgery, often fail in advanced stages, underscoring the urgent need for targeted interventions. TP-0903 emerges as a promising candidate in this landscape, functioning as a potent inhibitor of the AXL receptor tyrosine kinase, a molecule implicated in tumor proliferation, metastasis, and drug resistance.
The study reveals that TP-0903’s cytotoxic effects are intimately linked to the induction of reactive oxygen species within neuroblastoma cells. ROS, while classically recognized for their dual role in signaling and oxidative damage, serve here as pivotal executioners of programmed cell death. By tipping the redox balance, TP-0903 creates an intracellular environment conducive to apoptosis, effectively overcoming cellular defenses that typically shield tumor cells from oxidative stress.
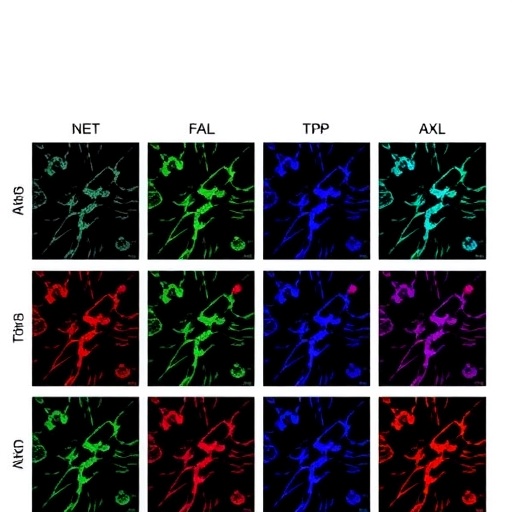
Central to this apoptotic cascade is the regulation of miR-335-3p, a microRNA previously uncharted in neuroblastoma pathology. MicroRNAs, short non-coding RNA sequences, regulate gene expression post-transcriptionally, thus orchestrating various biological processes including cell differentiation, proliferation, and apoptosis. The downregulation of miR-335-3p by TP-0903 is instrumental in modulating downstream gene targets, notably DKK1 (Dickkopf WNT Signaling Pathway Inhibitor 1), a gene that plays essential roles in Wnt signaling and cancer biology.
DKK1, acting as a modulator of the Wnt signaling pathway, is dysregulated in numerous cancers. In neuroblastoma, its expression is linked to cellular survival and proliferation. TP-0903-induced suppression of miR-335-3p precipitates an upregulation of DKK1, thereby altering the Wnt signaling dynamics and promoting apoptotic pathways. This nuanced molecular crosstalk underscores the complexity of intracellular signaling networks and the potential of modulating microRNA expression to achieve therapeutic effects.
The research further explores how AXL inhibition by TP-0903 disrupts oncogenic signaling cascades beyond ROS generation. AXL’s role extends to mediating tumor cell migration and evasion of immune surveillance, making it a multifaceted target. By attenuating AXL activity, TP-0903 not only triggers intrinsic apoptosis through oxidative mechanisms but may also impair neuroblastoma’s metastatic potential, suggesting a dual-pronged antitumor strategy.
In vitro analyses demonstrate that exposure of neuroblastoma cell lines to TP-0903 results in marked increases in ROS levels, which precede notable morphological and biochemical hallmarks of apoptosis, including mitochondrial membrane potential disruption and caspase activation. These findings validate the hypothesis that oxidative stress is not merely a byproduct but a central driver of cell death in this context.
Moreover, the modulation of miR-335-3p and consequent alterations in DKK1 expression are shown to be indispensable for TP-0903’s apoptotic efficacy. Experimental downregulation of miR-335-3p mimics the effects of TP-0903 treatment, while overexpression of miR-335-3p mitigates ROS induction and cell death, indicating a tightly controlled axis that could be leveraged therapeutically.
The implications of this research extend beyond neuroblastoma, as the involvement of AXL, ROS, and microRNA networks are common threads in diverse oncologic contexts. The therapeutic paradigm exemplified by TP-0903 could inform the design of multi-targeted kinase inhibitors, refined microRNA modulators, or combinatorial regimens that exploit vulnerabilities in tumor redox homeostasis and gene regulatory circuits.
Additionally, the study offers insights into the temporal dynamics of TP-0903’s effects, revealing a critical window during which ROS accumulation and miR-335-3p suppression converge to initiate irreversible apoptotic signaling. Understanding these kinetics is crucial for optimizing dosing regimens and minimizing potential adverse effects linked to oxidative damage in non-cancerous tissues.
This research represents a significant step forward in the exploitation of tyrosine kinase inhibitors within pediatric oncological therapeutics. The specificity of TP-0903 for AXL, combined with its capacity to modulate microRNA profiles and oxidative stress pathways, positions it as a promising agent that transcends traditional cytotoxic paradigms.
Clinical translation, while still in nascent stages, is anticipated to benefit greatly from these findings. Biomarker development centered on miR-335-3p and DKK1 expression levels could enable patient stratification and real-time monitoring of treatment response. Furthermore, integrating TP-0903 with existing treatment modalities holds promise for synergistic effects, potentially overcoming resistance mechanisms that have historically undermined neuroblastoma therapy.
Importantly, this study underscores the critical role of redox biology in cancer therapeutics. While excessive ROS are traditionally considered detrimental, their regulated induction, as leveraged by TP-0903, emerges as a powerful tool to selectively induce cancer cell apoptosis. This balances the potential toxicity with therapeutic benefit, a delicate interplay that demands further exploration in vivo.
The elucidation of the miR-335-3p/DKK1 axis not only advances our molecular understanding of neuroblastoma pathogenesis but also opens avenues for innovative RNA-based therapeutics. Synthetic mimics or inhibitors of specific microRNAs, in conjunction with kinase inhibitors, could provide tailored approaches with enhanced specificity and reduced off-target effects.
Future research will need to elaborate on the crosstalk between AXL signaling, ROS production, and microRNA modulation within the tumor microenvironment. Insights into how stromal components and immune cells are influenced by TP-0903 could unveil additional mechanisms by which tumor eradication can be achieved or enhanced.
In conclusion, the identification of TP-0903 as a potent inducer of ROS-mediated apoptosis via targeting the miR-335-3p/DKK1 pathway in neuroblastoma represents a paradigm shift in targeted cancer therapy. This multifaceted approach not only dismantles tumor survival strategies but also illuminates the complex molecular choreography underlying effective anticancer responses, heralding a new era of precision medicine for one of childhood’s deadliest malignancies.
Subject of Research: Neuroblastoma apoptosis mechanisms via AXL tyrosine kinase inhibition and microRNA modulation
Article Title: AXL tyrosine kinase inhibitor TP-0903 induces ROS trigger neuroblastoma cell apoptosis via targeting the miR-335-3p/DKK1 expression
Article References:
Tseng, TY., Kao, SH., Yang, SF. et al. AXL tyrosine kinase inhibitor TP-0903 induces ROS trigger neuroblastoma cell apoptosis via targeting the miR-335-3p/DKK1 expression. Cell Death Discov. 11, 378 (2025). https://doi.org/10.1038/s41420-025-02681-9
Image Credits: AI Generated